Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation
- PMID: 21282625
- PMCID: PMC3041089
- DOI: 10.1073/pnas.1013580108
Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation
Abstract
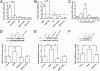
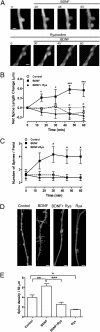
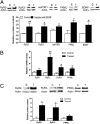
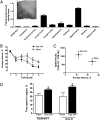
Ryanodine receptors (RyR) amplify activity-dependent calcium influx via calcium-induced calcium release. Calcium signals trigger postsynaptic pathways in hippocampal neurons that underlie synaptic plasticity, learning, and memory. Recent evidence supports a role of the RyR2 and RyR3 isoforms in these processes. Along with calcium signals, brain-derived neurotrophic factor (BDNF) is a key signaling molecule for hippocampal synaptic plasticity and spatial memory. Upon binding to specific TrkB receptors, BDNF initiates complex signaling pathways that modify synaptic structure and function. Here, we show that BDNF-induced remodeling of hippocampal dendritic spines required functional RyR. Additionally, incubation with BDNF enhanced the expression of RyR2, RyR3, and PKMζ, an atypical protein kinase C isoform with key roles in hippocampal memory consolidation. Consistent with their increased RyR protein content, BDNF-treated neurons generated larger RyR-mediated calcium signals than controls. Selective inhibition of RyR-mediated calcium release with inhibitory ryanodine concentrations prevented the PKMζ, RyR2, and RyR3 protein content enhancement induced by BDNF. Intrahippocampal injection of BDNF or training rats in a spatial memory task enhanced PKMζ, RyR2, RyR3, and BDNF hippocampal protein content, while injection of ryanodine at concentrations that stimulate RyR-mediated calcium release improved spatial memory learning and enhanced memory consolidation. We propose that RyR-generated calcium signals are key features of the complex neuronal plasticity processes induced by BDNF, which include increased expression of RyR2, RyR3, and PKMζ and the spine remodeling required for spatial memory formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Finkbeiner S, et al. CREB: A major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

