Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury
- PMID: 21283009
- PMCID: PMC3260884
- DOI: 10.1097/CCM.0b013e318207ec3c
Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury
Abstract
Objective: Multiple single biomarkers have been associated with poor outcomes in acute lung injury; however, no single biomarker has sufficient discriminating power to clearly indicate prognosis. Using both derivation and replication cohorts, we tested novel risk reclassification methods to determine whether measurement of multiple plasma biomarkers at the time of acute lung injury diagnosis would improve mortality prediction in acute lung injury.
Design: Analysis of plasma biomarker levels and prospectively collected clinical data from patients enrolled in two randomized controlled trials of ventilator therapy for acute lung injury.
Setting: Intensive care units of university hospitals participating in the National Institutes of Health Acute Respiratory Distress Syndrome Network.
Patients: Subjects enrolled in a trial of lower tidal volume ventilation (derivation cohort) and subjects enrolled in a trial of higher vs. lower positive end-expiratory pressure (replication cohort).
Interventions: None.
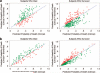
Measurements and main results: The plasma biomarkers were intercellular adhesion molecule-1, von Willebrand factor, interleukin-8, soluble tumor necrosis factor receptor-1, and surfactant protein-D. In the derivation cohort (n = 547), adding data on these biomarkers to clinical predictors (Acute Physiology and Chronic Health Evaluation III score) at the time of study enrollment improved the accuracy of risk prediction, as reflected by a net reclassification improvement of 22% (95% confidence interval 13% to 32%; p < .001). In the replication cohort (n = 500), the net reclassification improvement was 17% (95% confidence interval 7% to 26%; p < .001). A reduced set of three biomarkers (interleukin-8, soluble tumor necrosis factor receptor-1, and surfactant protein-D) had nearly equivalent prognostic value in both cohorts.
Conclusions: When combined with clinical data, plasma biomarkers measured at the onset of acute lung injury can improve the accuracy of risk prediction. Combining three or more biomarkers may be useful for selecting a high-risk acute lung injury population for enrollment in clinical trials of novel therapies.
Figures
References
-
- Ware LB. Prognostic determinants of acute respiratory distress syndrome in adults: Impact on clinical trial design. Crit Care Med. 2005;33:S217–S222. - PubMed
-
- Ware LB, Eisner MD, Thompson BT, et al. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. - PubMed
-
- Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. discussion 230-232. - PubMed
-
- Parsons PE, Matthay MA, Ware LB, et al. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L426–L431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 46057/PHS HHS/United States
- N01 HR046054/HL/NHLBI NIH HHS/United States
- 46055/PHS HHS/United States
- 46056/PHS HHS/United States
- HL090833/HL/NHLBI NIH HHS/United States
- KL2 RR024130/RR/NCRR NIH HHS/United States
- R01 HL051856/HL/NHLBI NIH HHS/United States
- 46060/PHS HHS/United States
- 46064/PHS HHS/United States
- R37 HL051856/HL/NHLBI NIH HHS/United States
- 46061/PHS HHS/United States
- N01-HR 46054/HR/NHLBI NIH HHS/United States
- HL 51856/HL/NHLBI NIH HHS/United States
- K24 HL103836/HL/NHLBI NIH HHS/United States
- 46063/PHS HHS/United States
- 46059/PHS HHS/United States
- 46058/PHS HHS/United States
- KL2RR024130/RR/NCRR NIH HHS/United States
- U01 HL081332/HL/NHLBI NIH HHS/United States
- HL081332/HL/NHLBI NIH HHS/United States
- 46062/PHS HHS/United States
- K23 HL090833/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

