Effects of non-thermal plasma on mammalian cells
- PMID: 21283714
- PMCID: PMC3025030
- DOI: 10.1371/journal.pone.0016270
Effects of non-thermal plasma on mammalian cells
Abstract
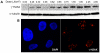
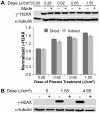
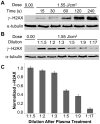
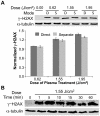
Thermal plasmas and lasers have been widely used in medicine to cut, ablate and cauterize tissues through heating; in contrast, non-thermal plasma produces no heat, so its effects can be selective. In order to exploit the potential for clinical applications, including wound healing, sterilization, blood coagulation, and cancer treatment, a mechanistic understanding of the interaction of non-thermal plasma with living tissues is required. Using mammalian cells in culture, it is shown here that non-thermal plasma created by dielectric barrier discharge (DBD) has dose-dependent effects that range from increasing cell proliferation to inducing apoptosis. It is also shown that these effects are primarily due to formation of intracellular reactive oxygen species (ROS). We have utilized γ-H2AX to detect DNA damage induced by non-thermal plasma and found that it is initiated by production of active neutral species that most likely induce formation of organic peroxides in cell medium. Phosphorylation of H2AX following non-thermal plasma treatment is ATR dependent and ATM independent, suggesting that plasma treatment may lead to replication arrest or formation of single-stranded DNA breaks; however, plasma does not lead to formation of bulky adducts/thymine dimers.
Conflict of interest statement
Figures




References
-
- Vargo JJ. Clinical applications of the argon plasma coagulator. Gastrointest Endosc. 2004;59:81–88. - PubMed
-
- Sladek REJ, Stoffels E. Deactivation of Escherichia coli by the plasma needle. Journal of Physics D: Applied Physics. 2005;38:1716–1721.
-
- Goree J, Bin l, Drake D, Stoffels E. Killing of S. mutans Bacteria Using a Plasma Needle at Atmospheric Pressure. IEEE Transactions on Plasma Science. 2006;34:1317–1324.
-
- Stoffels E, Kieft AIE, Sladek AREJ, Zandvoort V, Slaaf DW. Cold gas plasma in biology and medicine. In: d'Agostino R, Favia P, Kawai Y, Ikegami H, Sato N, et al., editors. Advanced Plasma Technology. Weinheim: Wiley-VCH; 2008. pp. 301–318.
-
- Stoffels E. Gas plasmas in biology and medicine. Journal of Physics D: Applied Physics. 2006;39
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

