Imaging of functional connectivity in the mouse brain
- PMID: 21283729
- PMCID: PMC3024435
- DOI: 10.1371/journal.pone.0016322
Imaging of functional connectivity in the mouse brain
Abstract
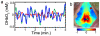
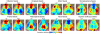
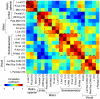
Functional neuroimaging (e.g., with fMRI) has been difficult to perform in mice, making it challenging to translate between human fMRI studies and molecular and genetic mechanisms. A method to easily perform large-scale functional neuroimaging in mice would enable the discovery of functional correlates of genetic manipulations and bridge with mouse models of disease. To satisfy this need, we combined resting-state functional connectivity mapping with optical intrinsic signal imaging (fcOIS). We demonstrate functional connectivity in mice through highly detailed fcOIS mapping of resting-state networks across most of the cerebral cortex. Synthesis of multiple network connectivity patterns through iterative parcellation and clustering provides a comprehensive map of the functional neuroarchitecture and demonstrates identification of the major functional regions of the mouse cerebral cortex. The method relies on simple and relatively inexpensive camera-based equipment, does not require exogenous contrast agents and involves only reflection of the scalp (the skull remains intact) making it minimally invasive. In principle, fcOIS allows new paradigms linking human neuroscience with the power of molecular/genetic manipulations in mouse models.
Conflict of interest statement
Figures



Comment in
-
The real-time technicolour living brain.Nature. 2016 Nov 10;539(7628):315-318. doi: 10.1038/539315a. Nature. 2016. PMID: 27830796 No abstract available.
References
-
- Raichle ME, Mintun MA. Brain work and brain imaging. The Annual Review of Neuroscience. 2006;29:449–476. - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. - PubMed
-
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

