Reasons for ineligibility in phase 1 and 2A HIV vaccine clinical trials at Kenya AIDS vaccine initiative (KAVI), Kenya
- PMID: 21283743
- PMCID: PMC3024980
- DOI: 10.1371/journal.pone.0014580
Reasons for ineligibility in phase 1 and 2A HIV vaccine clinical trials at Kenya AIDS vaccine initiative (KAVI), Kenya
Abstract
Background: With the persistent challenges towards controlling the HIV epidemic, there is an ongoing need for research into HIV vaccines and drugs. Sub-Saharan African countries--worst affected by the HIV pandemic--have participated in the conduct of clinical trials for HIV vaccines. In Kenya, the Kenya AIDS Vaccine Initiative (KAVI) at the University of Nairobi has conducted HIV vaccine clinical trials since 2001.
Methodology: Participants were recruited after an extensive informed consent process followed by screening to determine eligibility. Screening included an assessment of risk behavior, medical history and physical examination, and if clinically healthy, laboratory testing. In the absence of locally derived laboratory reference ranges, the ranges used in these trials were derived from populations in the West.
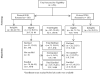
Principal findings: Two hundred eighty-one participants were screened between 2003 and 2006 for two clinical trials. Of these, 167 (59.4%) met the inclusion/exclusion criteria. Overall, laboratory abnormalities based on the non-indigenous laboratory references used were the most frequent reasons (61.4%) for ineligibility. Medical abnormalities contributed 30.7% of the total reasons for ineligibility. Based on the laboratory reference intervals now developed from East and Southern Africa, those ineligible due to laboratory abnormalities would have been 46.3%. Of the eligible participants, 18.6% declined enrollment.
Conclusions: Participant recruitment for HIV vaccine clinical trials is a rigorous and time-consuming exercise. Over 61% of the screening exclusions in clinically healthy people were due to laboratory abnormalities. It is essential that laboratory reference ranges generated from local populations for laboratory values be used in the conduct of clinical trials to avoid unnecessary exclusion of willing participants and to avoid over-reporting of adverse events for enrolled participants.
Trial registration: Protocol IAVI VRC V001 [1]. ClinicalTrials.gov NCT00124007 Protocol IAVI 010 [2](registration with ClincalTrials.gov is in progress) Protocols IAVI 002 and IAVI 004 are Phase 1 trials only mentioned in introductory paragraphs; details will not be reported. Registration was not required when they were conducted.
Conflict of interest statement
Figures
References
-
- Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007;25:2120–2127. - PubMed
-
- UNAIDS. Geneva; 2010. Report on the global HIV/AIDS epidemic.
-
- Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, et al. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine. 2008;26:2788–2795. - PubMed
-
- Omosa G, Jaoko W, Wakasiaka S, Ogutu H, Bwayo J. Baltimore, Maryland, USA: 5th Annual Conference on Vaccine Research; 2002. Volunteer Recruitment for phase 1 HIV Vaccine Trials in Nairobi.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous