Photothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystals
- PMID: 21284398
- PMCID: PMC3043169
- DOI: 10.1021/nn103415x
Photothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystals
Abstract
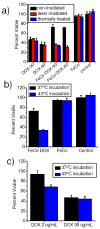
FeCo/graphitic carbon shell (FeCo/GC) nanocrystals (∼4-5 nm in diameter) with ultrahigh magnetization are synthesized, functionalized, and developed into multifunctional biocompatible materials. We demonstrate the ability of this material to serve as an integrated system for combined drug delivery, near-infrared (NIR) photothermal therapy, and magnetic resonance imaging (MRI) in vitro. We show highly efficient loading of doxorubicin (DOX) by π-stacking on the graphitic shell to afford FeCo/GC-DOX complexes and pH sensitive DOX release from the particles. We observe enhanced intracellular drug delivery by FeCo/GC-DOX under 20 min of NIR laser (808 nm) induced hyperthermia to 43 °C, resulting in a significant increase of FeCo/GC-DOX toxicity toward breast cancer cells. The synergistic cancer cell killing by FeCo/GC-DOX drug delivery under photothermal heating is due to a ∼two-fold enhancement of cancer cell uptake of FeCo/GC-DOX complex and the increased DOX toxicity under the 43 °C hyperthermic condition. The combination of synergistic NIR photothermally enhanced drug delivery and MRI with the FeCo/GC nanocrystals could lead to a powerful multimodal system for biomedical detection and therapy.
Figures



References
-
- Purushotham S, Chang PEJ, Rumpel H, Kee IHC, Ng RTH, Chow PKH, Tan CK, Ramanujan RV. Thermoresponsive Core-Shell Magnetic Nanoparticles for Combined Modalities of Cancer Therapy. Nanotechnology. 2009;20:305101. - PubMed
-
- Maeng JH, Lee DH, Jung KH, Bae YH, Park IS, Jeong S, Jeon YS, Shim CK, Kim W, Kim J, et al. Multifunctional Doxorubicin Loaded Superparamagnetic Iron Oxide Nanoparticles for Chemotherapy and Magnetic Resonance Imaging in Liver Cancer. Biomaterials. 2010;31:4995–5006. - PubMed
-
- Park H, Yang J, Lee J, Haam S, Choi IH, Yoo KH. Multifunctional Nanoparticles for Combined Doxorubicin and Photothermal Treatments. ACS Nano. 2009;3:2919–2926. - PubMed
-
- Lee SM, Park H, Yoo KH. Synergistic Cancer Therapeutic Effects of Locally Delivered Drug and Heat Using Multifunctional Nanoparticles. Adv Mater. 2010;22:4049–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

