Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations--twice daily Prograf and once daily Advagraf
- PMID: 21284698
- PMCID: PMC3045548
- DOI: 10.1111/j.1365-2125.2010.03837.x
Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations--twice daily Prograf and once daily Advagraf
Abstract
Aim: To investigate the differences in the pharmacokinetics of Prograf and the prolonged release formulation Advagraf and to develop a Bayesian estimator to estimate tacrolimus inter-dose area under the curve (AUC) in renal transplant patients receiving either Prograf or Advagraf.
Methods: Tacrolimus concentration-time profiles were collected, in adult renal transplant recipients, at weeks 1 and 2, and at months 1, 3 and 6 post-transplantation from 32 Prograf treated patients, and one profile was collected from 41 Advagraf patients more than 12 months post-transplantation. Population pharmacokinetic (popPK) parameters were estimated using nonmem. In a second step, the popPK model was used to develop a single Bayesian estimator for the two tacrolimus formulations.
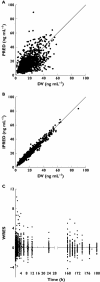
Results: A two-compartment model with Erlang absorption (n= 3) and first-order elimination best described the data. In Advagraf patients, a bimodal distribution was observed for the absorption rate constant (K(tr) ): one group with a K(tr) similar to that of Prograf treated patients and the other group with a slower absorption. A mixture model for K(tr) was tested to describe this bimodal distribution. However, the data were best described by the nonmixture model including covariates (cytochrome P450 3A5, haematocrit and drug formulation). Using this model and tacrolimus concentrations measured at 0, 1 and 3h post-dose, the Bayesian estimator could estimate tacrolimus AUC accurately (bias = 0.1%) and with good precision (8.6%).
Conclusions: The single Bayesian estimator developed yields good predictive performance for estimation of individual tacrolimus inter-dose AUC in Prograf and Advagraf treated patients and is suitable for clinical practice.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures

 ); Prograf (
); Prograf ( )
)


References
-
- Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G, Starzl T. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404–30. - PubMed
-
- Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, Abrams JD, Kamoun M, Rosas SE, Mange KC, Strom BL, Brayman KL, Feldman HI. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. 2005;16:1839–48. - PubMed
-
- Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900–5. - PubMed
-
- Silva HT. Tacrolimus once-daily formulation in the prophylaxis of transplant rejection in renal or liver allograft recipients: a viewpoint by Helio Tedesco Silva Jr. Drugs. 2007;67:1944–5. - PubMed
-
- Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, Hariharan S, Pirsch J, Matas A, Zaltzman J, Wisemandle K, Fitzsimmons W, First MR. Two years postconversion from a Prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients. Transplantation. 2007;83:1648–51. - PubMed

