Riluzole pharmacokinetics in young patients with spinal muscular atrophy
- PMID: 21284699
- PMCID: PMC3045549
- DOI: 10.1111/j.1365-2125.2010.03843.x
Riluzole pharmacokinetics in young patients with spinal muscular atrophy
Abstract
Aims: The objective of the present study was to assess the pharmacokinetics of riluzole in patients with spinal muscular atrophy (SMA).
Methods: Fourteen patients were enrolled in an open-label, nonrandomized and repeat-dose pharmacokinetic study. All participants were assigned to receive 50mg riluzole orally for 5 days. Riluzole plasma concentrations were determined from samples obtained at day 5.
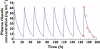
Results: The pharmacokinetic analysis demonstrated that a dose of 50mg once a day was sufficient to obtain a daily total exposure [AUC(0,24h)=2257ng ml(-1) h] which was comparable with results obtained in adult healthy volunteers or ALS patients in whom a dose of 50mg twice a day is recommended. The pharmacokinetic simulation demonstrated that the administration of 50mg twice a day could result in higher concentrations, hence reduced safety margin.
Conclusion: The dose of 50mg once a day was chosen for the clinical trial evaluating the efficacy of riluzole in SMA patients.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
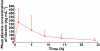
 ); Predicted (
); Predicted ( )
)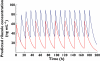
 ); twice daily (
); twice daily ( )
)References
-
- Mostacciuolo ML, Danieli GA, Trevisan C, Muller E, Angelini C. Epidemiology of spinal muscular atrophies in a sample of the Italian population. Neuroepidemiology. 1992;11:34–8. - PubMed
-
- Munsat TL, Skerry L, Korf B, Pober B, Schapira Y, Gascon GG, al-Rajeh SM, Dubowitz V, Davies K, Brzustowicz LM. Phenotypic heterogeneity of spinal muscular atrophy mapping to chromosome 5q11.2-13.3 (SMA 5q) Neurology. 1990;40:1831–6. - PubMed
-
- Greensmith L, Vrbova G. Possible strategies for treatment of SMA patients: a neurobiologist's view. Neuromuscul Disord. 1995;5:359–69. - PubMed
-
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–91. - PubMed
-
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

