Purification and biochemical characterization of a secreted group IIA chicken intestinal phospholipase A₂
- PMID: 21284884
- PMCID: PMC3040156
- DOI: 10.1186/1476-511X-10-27
Purification and biochemical characterization of a secreted group IIA chicken intestinal phospholipase A₂
Abstract
Background: Secretory phospholipase A2 group IIA (IIA PLA2) is a protein shown to be highly expressed in the intestine of mammals. However, no study was reported in birds.
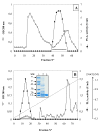
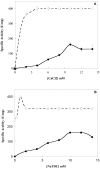
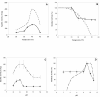
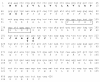
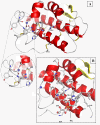
Results: Chicken intestinal group IIA phospholipase A₂ (ChPLA₂-IIA) was obtained after an acidic treatment (pH.3.0), precipitation by ammonium sulphate, followed by sequential column chromatographies on Sephadex G-50 and mono-S ion exchanger. The enzyme was found to be a monomeric protein with a molecular mass of around 14 kDa. The purified enzyme showed a substrate preference for phosphatidylethanolamine and phosphatidylglycerol, and didn't hydrolyse phosphatidylcholine. Under optimal assay conditions, in the presence of 10 mM NaTDC and 10 mM CaCl₂, a specific activity of 160 U.mg⁻¹ for purified ChPLA₂-IIA was measured using egg yolk as substrate. The fifteen NH2-terminal amino acid residues of ChPLA₂-IIA were sequenced and showed a close homology with known intestinal secreted phospholipases A₂. The gene encoding the mature ChPLA₂-IIA was cloned and sequenced. To further investigate structure-activity relationship, a 3D model of ChPLA₂-IIA was built using the human intestinal phospholipase A₂ structure as template.
Conclusion: ChPLA2-IIA was purified to homogeneity using only two chromatographic colomns. Sequence analysis of the cloned cDNA indicates that the enzyme is highly basic with a pI of 9.0 and has a high degree of homology with mammalian intestinal PLA₂-IIA.
Figures

References
-
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488(1-2):1–19. - PubMed
-
- Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A(2) and their receptors and binding proteins. Biochim Biophys Acta. 2000;1488(1-2):59–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

