Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle
- PMID: 21284933
- PMCID: PMC3101293
- DOI: 10.1016/j.abb.2011.01.017
Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle
Abstract
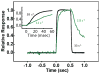
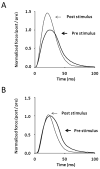
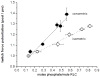
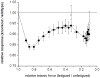
Skeletal muscle myosin light chain kinase (skMLCK) is a dedicated Ca(2+)/calmodulin-dependent serine-threonine protein kinase that phosphorylates the regulatory light chain (RLC) of sarcomeric myosin. It is expressed from the MYLK2 gene specifically in skeletal muscle fibers with most abundance in fast contracting muscles. Biochemically, activation occurs with Ca(2+) binding to calmodulin forming a (Ca(2+))(4)•calmodulin complex sufficient for activation with a diffusion limited, stoichiometric binding and displacement of a regulatory segment from skMLCK catalytic core. The N-terminal sequence of RLC then extends through the exposed catalytic cleft for Ser15 phosphorylation. Removal of Ca(2+) results in the slow dissociation of calmodulin and inactivation of skMLCK. Combined biochemical properties provide unique features for the physiological responsiveness of RLC phosphorylation, including (1) rapid activation of MLCK by Ca(2+)/calmodulin, (2) limiting kinase activity so phosphorylation is slower than contraction, (3) slow MLCK inactivation after relaxation and (4) much greater kinase activity relative to myosin light chain phosphatase (MLCP). SkMLCK phosphorylation of myosin RLC modulates mechanical aspects of vertebrate skeletal muscle function. In permeabilized skeletal muscle fibers, phosphorylation-mediated alterations in myosin structure increase the rate of force-generation by myosin cross bridges to increase Ca(2+)-sensitivity of the contractile apparatus. Stimulation-induced increases in RLC phosphorylation in intact muscle produces isometric and concentric force potentiation to enhance dynamic aspects of muscle work and power in unfatigued or fatigued muscle. Moreover, RLC phosphorylation-mediated enhancements may interact with neural strategies for human skeletal muscle activation to ameliorate either central or peripheral aspects of fatigue.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

