The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth
- PMID: 21284981
- PMCID: PMC3072597
- DOI: 10.1016/j.cmet.2011.01.005
The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth
Abstract
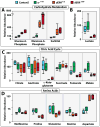
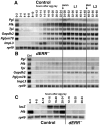
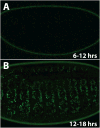
Metabolism must be coordinated with development to provide the appropriate energetic needs for each stage in the life cycle. Little is known, however, about how this temporal control is achieved. Here, we show that the Drosophila ortholog of the estrogen-related receptor (ERR) family of nuclear receptors directs a critical metabolic transition during development. dERR mutants die as larvae with low ATP levels and elevated levels of circulating sugars. The expression of active dERR protein in mid-embryogenesis triggers a coordinate switch in gene expression that drives a metabolic program normally associated with proliferating cells, supporting the dramatic growth that occurs during larval development. This study shows that dERR plays a central role in carbohydrate metabolism, demonstrates that a proliferative metabolic program is used in normal developmental growth, and provides a molecular context to understand the close association between mammalian ERR family members and cancer.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Abu-Shumays RL, Fristrom JW. IMP-L3, A 20-hydroxyecdysone-responsive gene encodes Drosophila lactate dehydrogenase: structural characterization and developmental studies. Dev Genet. 1997;20:11–22. - PubMed
-
- Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. - PubMed
-
- Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor alpha and estrogen-related receptor gamma associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res. 2002;62:6510–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

