Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis
- PMID: 21284986
- PMCID: PMC3033043
- DOI: 10.1016/j.cmet.2011.01.010
Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis
Abstract
Melanocortin-4 receptor (MC4R) mutations cause dysregulation of energy balance and hyperinsulinemia. We have used mouse models to study the physiological roles of extrahypothalamic MC4Rs. Re-expression of MC4Rs in cholinergic neurons (ChAT-Cre, loxTB MC4R mice) modestly reduced body weight gain without altering food intake and was sufficient to normalize energy expenditure and attenuate hyperglycemia and hyperinsulinemia. In contrast, restoration of MC4R expression in brainstem neurons including those in the dorsal motor nucleus of the vagus (Phox2b-Cre, loxTB MC4R mice) was sufficient to attenuate hyperinsulinemia, while the hyperglycemia and energy balance were not normalized. Additionally, hepatic insulin action and insulin-mediated suppression of hepatic glucose production were improved in ChAT-Cre, loxTB MC4R mice. These findings suggest that MC4Rs expressed by cholinergic neurons regulate energy expenditure and hepatic glucose production. Our results also provide further evidence of the dissociation in pathways mediating the effects of melanocortins on energy balance and glucose homeostasis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
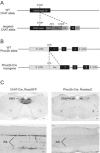

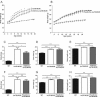


Comment in
-
Autonomic MC sets the metabolic tone.Cell Metab. 2011 Feb 2;13(2):121-3. doi: 10.1016/j.cmet.2011.01.014. Cell Metab. 2011. PMID: 21284978
References
-
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. - PubMed
-
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. - PubMed
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. - PubMed
-
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J Physiol. 1999;276:R1569–R1578. - PubMed
-
- Berthoud HR, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am. J. Physiol. 1990;258:R523–R530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- TL1 DK081181/DK/NIDDK NIH HHS/United States
- DK071320/DK/NIDDK NIH HHS/United States
- RL1 DK081185/DK/NIDDK NIH HHS/United States
- K08 DK071561/DK/NIDDK NIH HHS/United States
- R37 DK053301/DK/NIDDK NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- RL1DK081185/DK/NIDDK NIH HHS/United States
- R01 DK053301/DK/NIDDK NIH HHS/United States
- UL1 DE019584/DE/NIDCR NIH HHS/United States
- R01 DK056690/DK/NIDDK NIH HHS/United States
- R01 DK075632/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R56 DK071320/DK/NIDDK NIH HHS/United States
- K99DK024719-02/DK/NIDDK NIH HHS/United States
- FS/06/057/BHF_/British Heart Foundation/United Kingdom
- R01DK53301/DK/NIDDK NIH HHS/United States
- K08DK071561/DK/NIDDK NIH HHS/United States
- 5TL1DK081181/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- K99 DA024719/DA/NIDA NIH HHS/United States
- P30DK046200/DK/NIDDK NIH HHS/United States
- R01DK075632/DK/NIDDK NIH HHS/United States
- UL1 RR024923/RR/NCRR NIH HHS/United States
- PL1 DK081182/DK/NIDDK NIH HHS/United States
- R01 DK071320/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

