Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase
- PMID: 21284988
- PMCID: PMC3042716
- DOI: 10.1016/j.cmet.2011.01.007
Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase
Abstract
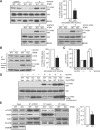
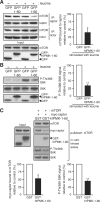
mTOR complex 1 (mTORC1; mammalian target of rapamycin [mTOR] in complex with raptor) is a key regulator of protein synthesis and cell growth in response to nutrient amino acids. Here we report that inositol polyphosphate multikinase (IPMK), which possesses both inositol phosphate kinase and lipid kinase activities, regulates amino acid signaling to mTORC1. This regulation is independent of IPMK's catalytic function, instead reflecting its binding with mTOR and raptor, which maintains the mTOR-raptor association. Thus, IPMK appears to be a physiologic mTOR cofactor, serving as a determinant of mTORC1 stability and amino acid-induced mTOR signaling. Substances that block IPMK-mTORC1 binding may afford therapeutic benefit in nutrient amino acid-regulated conditions such as obesity and diabetes.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Nutrient amino acids signal to mTOR via inositol polyphosphate multikinase.Cell Cycle. 2011 Jun 1;10(11):1708-10. doi: 10.4161/cc.10.11.15559. Epub 2011 Jun 1. Cell Cycle. 2011. PMID: 21633199 No abstract available.
References
-
- Béchet J, Grenson M, Wiame JM. Mutations affecting the repressibility of arginine biosynthetic enzymes in Sacchromyces cerevisiae. Eur. J. Biochem. 1970;12:31–39. - PubMed
-
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. - PubMed
-
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 2007;13:252–259. - PubMed
-
- Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. - PubMed
-
- Dubois E, Dewaste V, Erneux C, Messenguy F. Inositol polyphosphate kinase activity of Arg82/ArgRIII is not required for the regulation of the arginine metabolism in yeast. FEBS. Lett. 2000;486:300–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

