Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists
- PMID: 21285282
- PMCID: PMC3083103
- DOI: 10.1124/jpet.110.177485
Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists
Abstract
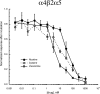
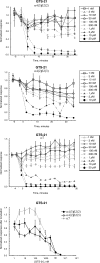
Partial agonist therapies rely variously on two hypotheses: the partial agonists have their effects through chronic low-level receptor activation or the partial agonists work by decreasing the effects of endogenous or exogenous full agonists. The relative significance of these activities probably depends on whether acute or chronic effects are considered. We studied nicotinic acetylcholine receptors (nAChRs) expressed in Xenopus laevis oocytes to test a model for the acute interactions between acetylcholine (ACh) and weak partial agonists. Data were best-fit to a basic competition model that included an additional factor for noncompetitive inhibition. Partial agonist effects were compared with the nAChR antagonist bupropion in prolonged bath application experiments that were designed to mimic prolonged drug exposure typical of therapeutic drug delivery. A primary effect of prolonged application of nicotine was to decrease the response of all nAChR subtypes to acute applications of ACh. In addition, nicotine, cytisine, and varenicline produced detectable steady-state activation of α4β2* [(α4)(2)(β2)(3), (α4)(3)(β2)(2), and (α4)(2)(β2)(2)α5)] receptor subtypes that was not seen with other test compounds. Partial agonists produced no detectable steady-state activation of α7 nAChR, but seemed to show small potentiation of ACh-evoked responses; however, "run-up" of α7 ACh responses was also sometimes observed under control conditions. Potential off-target effects of the partial agonists therefore included the modulation of α7 responses by α4β2 partial agonists and decreases in α4β2* responses by α7-selective agonists. These data indicate the dual effects expected for α4β2* partial agonists and provide models and insights for utility of partial agonists in therapeutic development.
Figures








References
-
- Bullock AE, Clark AL, Grady SR, Robinson SF, Slobe BS, Marks MJ, Collins AC. (1997) Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes. J Neurochem 68:2412–2423 - PubMed
-
- de Fiebre CM, Meyer EM, Henry JC, Muraskin SI, Kem WR, Papke RL. (1995) Characterization of a family of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic α7/[125I]α-bungarotoxin receptor subtypes. Mol Pharmacol 47:164–171 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

