Sexually dimorphic diet-induced insulin resistance in obese tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice
- PMID: 21285317
- PMCID: PMC3060627
- DOI: 10.1210/en.2010-1029
Sexually dimorphic diet-induced insulin resistance in obese tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice
Abstract
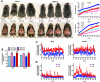
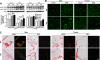
Circulating levels of matrix metalloproteinases (MMPs) and their endogenous inhibitors, tissue inhibitor of metalloproteinases (TIMPs), are altered in human obesity and may contribute to its pathology. TIMP-2 exerts MMP-dependent (MMP inhibition and pro-MMP-2 activation) and MMP-independent functions. To assess the role of TIMP-2 in a murine model of nutritionally induced obesity, weight gain in wild-type and TIMP-2 deficient [knockout (KO)] mice fed a chow or high-fat diet (HFD) was determined. The effects of diet on glucose tolerance and insulin sensitivity, as well as pancreatic β-cell and adipocyte physiology, were assessed. Chow-fed TIMP-2 KO mice of both sexes became obese but maintained relatively normal glucose tolerance and insulin sensitivity. Obesity was exacerbated on the HFD. However, HFD-fed male, but not female, TIMP-2 KO mice developed insulin resistance with reduced glucose transporter 2 and pancreatic and duodenal homeobox 1 levels, despite increased β-cell mass and hyperplasia. Thus, although β-cell mass was increased, HFD-fed male TIMP-2 KO mice develop diabetes likely due to β-cell exhaustion and failure. TIMP-2 mRNA, whose expression was greatest in sc adipose tissue, was down-regulated in HFD-fed wild-type males, but not females. Furthermore, HFD increased membrane type 1-MMP (MMP-14) expression and activity in male, but not female, sc adipose tissue. Strikingly, MMP-14 expression increased to a greater extent in TIMP-2 KO males and was associated with decreased adipocyte collagen. Taken together, these findings demonstrate a role for TIMP-2 in maintaining extracellular matrix integrity necessary for normal β-cell and adipocyte physiology and that loss of extracellular matrix integrity may underlie diabetic and obesogenic phenotypes.
Figures
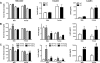



References
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. 2010. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241 - PubMed
-
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. 2010. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 303:242–249 - PubMed
-
- Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. 2003. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26:372–379 - PubMed
-
- Yki-Järvinen H. 2005. Fat in the liver and insulin resistance. Ann Med 37:347–356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

