Protein kinase D negatively regulates hepatitis C virus secretion through phosphorylation of oxysterol-binding protein and ceramide transfer protein
- PMID: 21285358
- PMCID: PMC3064182
- DOI: 10.1074/jbc.M110.182097
Protein kinase D negatively regulates hepatitis C virus secretion through phosphorylation of oxysterol-binding protein and ceramide transfer protein
Abstract
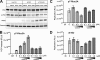
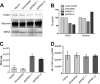
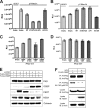
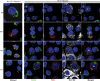
Hepatitis C virus (HCV) RNA replicates its genome on specialized endoplasmic reticulum modified membranes termed membranous web and utilizes lipid droplets for initiating the viral nucleocapsid assembly. HCV maturation and/or the egress pathway requires host sphingolipid synthesis, which occur in the Golgi. Ceramide transfer protein (CERT) and oxysterol-binding protein (OSBP) play a crucial role in sphingolipid biosynthesis. Protein kinase D (PKD), a serine/threonine kinase, is recruited to the trans-Golgi network where it influences vesicular trafficking to the plasma membrane by regulation of several important mediators via phosphorylation. PKD attenuates the function of both CERT and OSBP by phosphorylation at their respective Ser(132) and Ser(240) residues (phosphorylation inhibition). Here, we investigated the functional role of PKD in HCV secretion. Our studies show that HCV gene expression down-regulated PKD activation. PKD depletion by shRNA or inhibition by pharmacological inhibitor Gö6976 enhanced HCV secretion. Overexpression of a constitutively active form of PKD suppressed HCV secretion. The suppression by PKD was subverted by the ectopic expression of nonphosphorylatable serine mutant CERT S132A or OSBP S240A. These observations imply that PKD negatively regulates HCV secretion/release by attenuating OSBP and CERT functions by phosphorylation inhibition. This study identifies the key role of the Golgi components in the HCV maturation process.
Figures


References
-
- Di Bisceglie A. M. (2002) J. Vasc. Interv. Radiol. 13, S169–171 - PubMed
-
- Pawlotsky J. M. (2004) Trends Microbiol. 12, 96–102 - PubMed
-
- Negro F., Sanyal A. J. (2009) Liver Int. 29, Suppl. 2, 26–37 - PubMed
-
- Appel N., Schaller T., Penin F., Bartenschlager R. (2006) J. Biol. Chem. 281, 9833–9836 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

