Wnt signaling regulates hepatic metabolism
- PMID: 21285411
- PMCID: PMC3147298
- DOI: 10.1126/scisignal.2001249
Wnt signaling regulates hepatic metabolism
Abstract
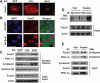
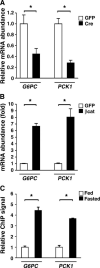
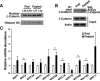
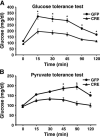
The contribution of the Wnt pathway has been extensively characterized in embryogenesis, differentiation, and stem cell biology but not in mammalian metabolism. Here, using in vivo gain- and loss-of-function models, we demonstrate an important role for Wnt signaling in hepatic metabolism. In particular, β-catenin, the downstream mediator of canonical Wnt signaling, altered serum glucose concentrations and regulated hepatic glucose production. β-Catenin also modulated hepatic insulin signaling. Furthermore, β-catenin interacted with the transcription factor FoxO1 in livers from mice under starved conditions. The interaction of FoxO1 with β-catenin regulated the transcriptional activation of the genes encoding glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK), the two rate-limiting enzymes in hepatic gluconeogenesis. Moreover, starvation induced the hepatic expression of mRNAs encoding different Wnt isoforms. In addition, nutrient deprivation appeared to favor the association of β-catenin with FoxO family members, rather than with members of the T cell factor of transcriptional activators. Notably, in a model of diet-induced obesity, hepatic deletion of β-catenin improved overall metabolic homeostasis. These observations implicate Wnt signaling in the modulation of hepatic metabolism and raise the possibility that Wnt signaling may play a similar role in the metabolic regulation of other tissues.
Figures




References
-
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. - PubMed
-
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. - PubMed
-
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. - PubMed
-
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

