Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies
- PMID: 21285439
- PMCID: PMC3099573
- DOI: 10.1182/blood-2010-07-295857
Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies
Abstract
Single nucleotide polymorphism arrays (SNP-As) have emerged as an important tool in the identification of chromosomal defects undetected by metaphase cytogenetics (MC) in hematologic cancers, offering superior resolution of unbalanced chromosomal defects and acquired copy-neutral loss of heterozygosity. Myelodysplastic syndromes (MDSs) and related cancers share recurrent chromosomal defects and molecular lesions that predict outcomes. We hypothesized that combining SNP-A and MC could improve diagnosis/prognosis and further the molecular characterization of myeloid malignancies. We analyzed MC/SNP-A results from 430 patients (MDS = 250, MDS/myeloproliferative overlap neoplasm = 95, acute myeloid leukemia from MDS = 85). The frequency and clinical significance of genomic aberrations was compared between MC and MC plus SNP-A. Combined MC/SNP-A karyotyping lead to higher diagnostic yield of chromosomal defects (74% vs 44%, P < .0001), compared with MC alone, often through detection of novel lesions in patients with normal/noninformative (54%) and abnormal (62%) MC results. Newly detected SNP-A defects contributed to poorer prognosis for patients stratified by current morphologic and clinical risk schemes. The presence and number of new SNP-A detected lesions are independent predictors of overall and event-free survival. The significant diagnostic and prognostic contributions of SNP-A-detected defects in MDS and related diseases underscore the utility of SNP-A when combined with MC in hematologic malignancies.
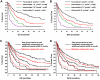
Figures



References
-
- Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325–4336. - PubMed
-
- Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–2333. - PubMed
-
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. - PubMed
-
- Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining [letter]. Nature. 1973;243(5405):290–293. - PubMed
-
- de The H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347(6293):558–561. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

