The biophysical and molecular basis of TRPV1 proton gating
- PMID: 21285946
- PMCID: PMC3061026
- DOI: 10.1038/emboj.2011.19
The biophysical and molecular basis of TRPV1 proton gating
Abstract
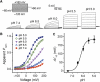
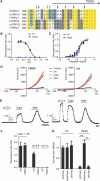
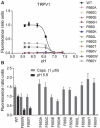
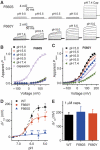
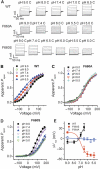
The capsaicin receptor TRPV1, a member of the transient receptor potential family of non-selective cation channels is a polymodal nociceptor. Noxious thermal stimuli, protons, and the alkaloid irritant capsaicin open the channel. The mechanisms of heat and capsaicin activation have been linked to voltage-dependent gating in TRPV1. However, until now it was unclear whether proton activation or potentiation or both are linked to a similar voltage-dependent mechanism and which molecular determinants underlie the proton gating. Using the whole-cell patch-clamp technique, we show that protons activate and potentiate TRPV1 by shifting the voltage dependence of the activation curves towards more physiological membrane potentials. We further identified a key residue within the pore region of TRPV1, F660, to be critical for voltage-dependent proton activation and potentiation. We conclude that proton activation and potentiation of TRPV1 are both voltage dependent and that amino acid 660 is essential for proton-mediated gating of TRPV1.
Conflict of interest statement
The authors declare that they have no conflict of interest. All authors are Pfizer employees.
Figures

References
-
- Chou MZ, Mtui T, Gao YD, Kohler M, Middleton RE (2004) Resiniferatoxin binds to the capsaicin receptor (TRPV1) near the extracellular side of the S4 transmembrane domain. Biochemistry 43: 2501–2511 - PubMed
-
- Clapham DE (2003) TRP channels as cellular sensors. Nature 426: 517–524 - PubMed
-
- Clapham DE, Montell C, Schultz G, Julius D (2003) International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev 55: 591–596 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

