A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification
- PMID: 21285948
- PMCID: PMC3049207
- DOI: 10.1038/emboj.2010.363
A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification
Abstract
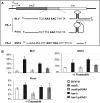
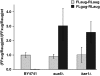
The YgjD/Kae1 family (COG0533) has been on the top-10 list of universally conserved proteins of unknown function for over 5 years. It has been linked to DNA maintenance in bacteria and mitochondria and transcription regulation and telomere homeostasis in eukaryotes, but its actual function has never been found. Based on a comparative genomic and structural analysis, we predicted this family was involved in the biosynthesis of N(6)-threonylcarbamoyl adenosine, a universal modification found at position 37 of tRNAs decoding ANN codons. This was confirmed as a yeast mutant lacking Kae1 is devoid of t(6)A. t(6)A(-) strains were also used to reveal that t(6)A has a critical role in initiation codon restriction to AUG and in restricting frameshifting at tandem ANN codons. We also showed that YaeZ, a YgjD paralog, is required for YgjD function in vivo in bacteria. This work lays the foundation for understanding the pleiotropic role of this universal protein family.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



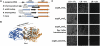

References
-
- Abdullah KM, Lo RY, Mellors A (1990) Distribution of glycoprotease activity and the glycoprotease gene among serotypes of Pasteurella haemolytica. Biochem Soc Trans 18: 901–903 - PubMed
-
- Arigoni F, Talabot F, Peitsch M, Edgerton MD, Meldrum E, Allet E, Fish R, Jamotte T, Curchod ML, Loferer H (1998) A genome-based approach for the identification of essential bacterial genes. Nat Biotechnol 16: 851–856 - PubMed
-
- Baba T, Mori H (2008) The construction of systematic in-frame, single-gene knockout mutant collection in Escherichia coli K-12. Methods Mol Biol 416: 171–181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

