T-wave alternans and arrhythmogenesis in cardiac diseases
- PMID: 21286254
- PMCID: PMC3028203
- DOI: 10.3389/fphys.2010.00154
T-wave alternans and arrhythmogenesis in cardiac diseases
Abstract
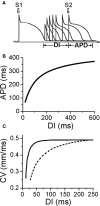
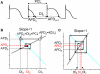
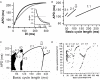
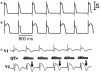
T-wave alternans, a manifestation of repolarization alternans at the cellular level, is associated with lethal cardiac arrhythmias and sudden cardiac death. At the cellular level, several mechanisms can produce repolarization alternans, including: 1) electrical restitution resulting from collective ion channel recovery, which usually occurs at fast heart rates but can also occur at normal heart rates when action potential is prolonged resulting in a short diastolic interval; 2) the transient outward current, which tends to occur at normal or slow heart rates; 3) the dynamics of early afterdepolarizations, which tends to occur during bradycardia; and 4) intracellular calcium cycling alternans through its interaction with membrane voltage. In this review, we summarize the cellular mechanisms of alternans arising from these different mechanisms, and discuss their roles in arrhythmogenesis in the setting of cardiac disease.
Keywords: T-wave alternans; afterdepolarizations; arrhythmias; calcium cycling; cardiac diseases; restitution.
Figures





References
-
- Antoons G., Volders P. G., Stankovicova T., Bito V., Stengl M., Vos M. A., Sipido K. R. (2007). Window Ca2+ current and its modulation by Ca2+ release in hypertrophied cardiac myocytes from dogs with chronic atrioventricular block. J. Physiol. 579, 147–16010.1113/jphysiol.2006.124222 - DOI - PMC - PubMed
-
- Armoundas A. A., Nanke T., Cohen R. J. (2000). Images in cardiovascular medicine. T-wave alternans preceding torsade de pointes ventricular tachycardia.Circulation 101, 2550. - PubMed

