Combinatorial Tau pseudophosphorylation: markedly different regulatory effects on microtubule assembly and dynamic instability than the sum of the individual parts
- PMID: 21288907
- PMCID: PMC3077627
- DOI: 10.1074/jbc.M111.219311
Combinatorial Tau pseudophosphorylation: markedly different regulatory effects on microtubule assembly and dynamic instability than the sum of the individual parts
Abstract
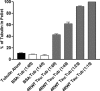
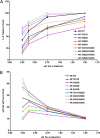
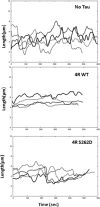
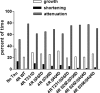
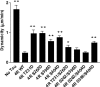
Tau is a multiply phosphorylated protein that is essential for the development and maintenance of the nervous system. Errors in Tau action are associated with Alzheimer disease and related dementias. A huge literature has led to the widely held notion that aberrant Tau hyperphosphorylation is central to these disorders. Unfortunately, our mechanistic understanding of the functional effects of combinatorial Tau phosphorylation remains minimal. Here, we generated four singly pseudophosphorylated Tau proteins (at Thr(231), Ser(262), Ser(396), and Ser(404)) and four doubly pseudophosphorylated Tau proteins using the same sites. Each Tau preparation was assayed for its abilities to promote microtubule assembly and to regulate microtubule dynamic instability in vitro. All four singly pseudophosphorylated Tau proteins exhibited loss-of-function effects. In marked contrast to the expectation that doubly pseudophosphorylated Tau would be less functional than either of its corresponding singly pseudophosphorylated forms, all of the doubly pseudophosphorylated Tau proteins possessed enhanced microtubule assembly activity and were more potent at regulating dynamic instability than their compromised singly pseudophosphorylated counterparts. Thus, the effects of multiple pseudophosphorylations were not simply the sum of the effects of the constituent single pseudophosphorylations; rather, they were generally opposite to the effects of singly pseudophosphorylated Tau. Further, despite being pseudophosphorylated at different sites, the four singly pseduophosphorylated Tau proteins often functioned similarly, as did the four doubly pseudophosphorylated proteins. These data lead us to reassess the conventional view of combinatorial phosphorylation in normal and pathological Tau action. They may also be relevant to the issue of combinatorial phosphorylation as a general regulatory mechanism.
Figures
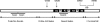
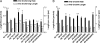
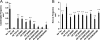
Similar articles
-
Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation.J Biol Chem. 2006 Jul 14;281(28):19107-14. doi: 10.1074/jbc.M511697200. Epub 2006 May 10. J Biol Chem. 2006. PMID: 16687396
-
Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration.J Biol Chem. 2010 Oct 1;285(40):30851-60. doi: 10.1074/jbc.M110.110957. Epub 2010 Jul 27. J Biol Chem. 2010. PMID: 20663882 Free PMC article.
-
Conformational changes specific for pseudophosphorylation at serine 262 selectively impair binding of tau to microtubules.Biochemistry. 2009 Oct 27;48(42):10047-55. doi: 10.1021/bi901090m. Biochemistry. 2009. PMID: 19769346
-
Tau domains, phosphorylation, and interactions with microtubules.Neurobiol Aging. 1995 May-Jun;16(3):355-62; discussion 362-3. doi: 10.1016/0197-4580(95)00025-a. Neurobiol Aging. 1995. PMID: 7566345 Review.
-
Quantitative analysis of MAP-mediated regulation of microtubule dynamic instability in vitro focus on Tau.Methods Cell Biol. 2010;95:481-503. doi: 10.1016/S0091-679X(10)95024-3. Methods Cell Biol. 2010. PMID: 20466149 Review.
Cited by
-
An experimental model of Braak's pretangle proposal for the origin of Alzheimer's disease: the role of locus coeruleus in early symptom development.Alzheimers Res Ther. 2019 Jul 3;11(1):59. doi: 10.1186/s13195-019-0511-2. Alzheimers Res Ther. 2019. PMID: 31266535 Free PMC article.
-
A novel MAPT mutation, G55R, in a frontotemporal dementia patient leads to altered Tau function.PLoS One. 2013 Sep 27;8(9):e76409. doi: 10.1371/journal.pone.0076409. eCollection 2013. PLoS One. 2013. PMID: 24086739 Free PMC article.
-
Tau antagonizes end-binding protein tracking at microtubule ends through a phosphorylation-dependent mechanism.Mol Biol Cell. 2016 Oct 1;27(19):2924-34. doi: 10.1091/mbc.E16-01-0029. Epub 2016 Jul 27. Mol Biol Cell. 2016. PMID: 27466319 Free PMC article.
-
The Effect of the Tau Protein on D. melanogaster Lifespan Depends on GSK3 Expression and Sex.Int J Mol Sci. 2023 Jan 21;24(3):2166. doi: 10.3390/ijms24032166. Int J Mol Sci. 2023. PMID: 36768490 Free PMC article.
-
Microtubule-associated protein tau in bovine retinal photoreceptor rod outer segments: comparison with brain tau.Biochim Biophys Acta. 2013 Oct;1832(10):1549-59. doi: 10.1016/j.bbadis.2013.05.021. Epub 2013 May 24. Biochim Biophys Acta. 2013. PMID: 23712071 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

