Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1
- PMID: 21289052
- PMCID: PMC3094059
- DOI: 10.1152/ajprenal.00353.2010
Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1
Abstract
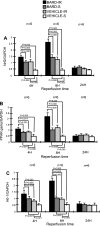
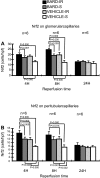
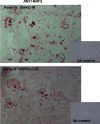
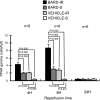
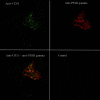
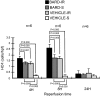
Ischemic acute kidney injury (AKI) triggers expression of adaptive (protective) and maladaptive genes. Agents that increase expression of protective genes should provide a therapeutic benefit. We now report that bardoxolone methyl (BARD) ameliorates ischemic murine AKI as assessed by both renal function and pathology. BARD may exert its beneficial effect by increasing expression of genes previously shown to protect against ischemic AKI, NF-E2-related factor 2 (Nrf2), peroxisome proliferator-activated receptor-γ (PPARγ), and heme oxygenase 1 (HO-1). Although we found that BARD alone or ischemia-reperfusion alone increased expression of these genes, the greatest increase occurred after the combination of both ischemia-reperfusion and BARD. BARD had a different mode of action than other agents that regulate PPARγ and Nrf2. Thus we report that BARD regulates PPARγ, not by acting as a ligand but by increasing the amount of PPARγ mRNA and protein. This should increase ligand-independent effects of PPARγ. Similarly, BARD increased Nrf2 mRNA; this increased Nrf2 protein by mechanisms in addition to the prolongation of Nrf2 protein half-life previously reported. Finally, we localized expression of these protective genes after ischemia and BARD treatment. Using double-immunofluorescence staining for CD31 and Nrf2 or PPARγ, we found increased Nrf2 and PPARγ on glomerular endothelia in the cortex; Nrf2 was also present on cortical peritubular capillaries. In contrast, HO-1 was localized to different cells, i.e., tubules and interstitial leukocytes. Although Nrf2-dependent increases in HO-1 have been described, our data suggest that BARD's effects on tubular and leukocyte HO-1 during ischemic AKI may be Nrf2 independent. We also found that BARD ameliorated cisplatin nephrotoxicity.
Figures










References
-
- Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappa B pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem 281: 35764–35769, 2006. - PubMed
-
- Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 36: 166–174, 2007. - PubMed
-
- Alvarez-Maqueda M, El Bekay R, Alba G, Monteseirin J, Chacon P, Vega A, Martin-Nieto J, Bedoya FJ, Pintado E, Sobrino F. 15-Deoxy-delta 12,14-prostaglandin J2 induces heme oxygenase-1 gene expression in a reactive oxygen species-dependent manner in human lymphocytes. J Biol Chem 279: 21929–21937, 2004. - PubMed
-
- Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 43: 993–1002, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

