Role of smooth muscle protein SM22α in glomerular epithelial cell injury
- PMID: 21289056
- PMCID: PMC3074994
- DOI: 10.1152/ajprenal.00187.2010
Role of smooth muscle protein SM22α in glomerular epithelial cell injury
Abstract
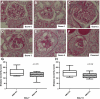
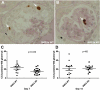
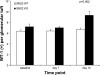
Podocytes are considered terminally differentiated cells in the mature kidney under normal conditions. In the face of injury, podocytes may proceed along several possible pathways, including dedifferentiation and proliferation, persistent cell cycle arrest, hypertrophy, apoptosis, or necrosis. There is mounting evidence that transdifferentiation into a dysregulated phenotype may also be a potential cell fate. We have previously reported that the transcript of SM22α, an actin-binding protein considered one of the earliest markers of smooth muscle differentiation, is upregulated nearly 70-fold in glomeruli of rats with passive Heymann nephritis (PHN). In contrast, the SM22α transcript is absent in normal adult rat glomeruli. The purpose of this study was to define SM22α's expression during kidney development and its role in glomerular diseases characterized by podocyte injury and proteinuria. During glomerulogenesis and podocyte differentiation, SM22α was expressed in glomeruli. This expression disappeared with glomerular maturation. Along with SM22α induction in PHN, confirmed at both mRNA and protein levels, SM22α was also induced across a broad range of proteinuric diseases, including experimental animal models (puromycin aminonucleoside nephropathy, adriamycin nephropathy, passive nephrotoxic nephritis, and diet-induced obesity) and human diseases (collapsing glomerulopathy, diabetic nephropathy, classic focal segmental glomerulosclerosis, IgA nephropathy, minimal-change disease, membranous nephropathy, and membranoproliferative glomerulonephritis). Crescentic glomerulonephritis was induced in SM22α +/+ and SM22α -/- mice by intraperitoneal injection of sheep anti-rabbit glomeruli antibody 12.5 mg/20 g body wt × 2 doses (n = 12-15/group), with mice euthanized at 7 and 14 days. Compared with SM22α -/- mice, SM22α +/+ mice demonstrated worse disease by histopathological parameters. In addition, there was greater apoptosis (cleaved caspase-3 immunostaining), fewer podocytes (Wilms' tumor-1 immunostaining), and less proliferation (Ki-67 immunostaining) in diseased SM22α +/+ mice. Furthermore, there was decreased activation of Erk1/2 in diseased SM22α +/+ mice. We conclude that the de novo expression of SM22α in glomerular epithelial cells affects the course of crescentic glomerulonephritis.
Figures









References
-
- Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J Biol Chem 275: 37798–37806, 2000 - PubMed
-
- Assinder SJ, Stanton JA, Prasad PD. Transgelin: an actin-binding protein and tumour suppressor. Int J Biochem Cell Biol 41: 482–486, 2009 - PubMed
-
- Bariety J, Bruneval P, Hill GS, Mandet C, Jacquot C, Meyrier A. Transdifferentiation of epithelial glomerular cells. J Am Soc Nephrol 14, Suppl 1: S42–S47, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

