Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1
- PMID: 21289128
- PMCID: PMC3126129
- DOI: 10.1128/JVI.02438-10
Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1
Abstract
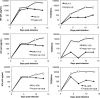
Among Old World monkeys, pig-tailed macaques (Pt) are uniquely susceptible to human immunodeficiency virus type 1 (HIV-1), although the infection does not persist. We demonstrate that the susceptibility of Pt T cells to HIV-1 infection is due to the absence of postentry inhibition by a TRIM5 isoform. Notably, substitution of the viral infectivity factor protein, Vif, with that from pathogenic SIVmne enabled replication of HIV-1 in Pt T cells in vitro. When inoculated into juvenile pig-tailed macaques, the Pt-tropic HIV-1 persistently replicated for more than 1.5 to 2 years, producing low but measurable plasma viral loads and persistent proviral DNA in peripheral blood mononuclear cells. It also elicited strong antibody responses. However, there was no decline in CD4(+) T cells or evidence of disease. Surprisingly, the Pt-tropic HIV-1 was rapidly controlled when inoculated into newborn Pt macaques, although it transiently rebounded after 6 months. We identified two notable differences between the Pt-tropic HIV-1 and SIVmne. First, SIV Vif does not associate with Pt-tropic HIV-1 viral particles. Second, while Pt-tropic HIV-1 degrades both Pt APOBEC3G and APOBEC3F, it prevents their inclusion in virions to a lesser extent than pathogenic SIVmne. Thus, while SIV Vif is necessary for persistent infection by Pt-tropic HIV-1, improved expression and inhibition of APOBEC3 proteins may be required for robust viral replication in vivo. Additional adaptation of the virus may also be necessary to enhance viral replication. Nevertheless, our data suggest the potential for the pig-tailed macaque to be developed as an animal model of HIV-1 infection and disease.
Figures



Similar articles
-
Enhanced infectivity of an R5-tropic simian/human immunodeficiency virus carrying human immunodeficiency virus type 1 subtype C envelope after serial passages in pig-tailed macaques (Macaca nemestrina).J Virol. 2000 Jul;74(14):6501-10. doi: 10.1128/jvi.74.14.6501-6510.2000. J Virol. 2000. PMID: 10864663 Free PMC article.
-
Mutations in the highly conserved SLQYLA motif of Vif in a simian-human immunodeficiency virus result in a less pathogenic virus and are associated with G-to-A mutations in the viral genome.Virology. 2009 Jan 20;383(2):362-72. doi: 10.1016/j.virol.2008.10.013. Epub 2008 Nov 21. Virology. 2009. PMID: 19027134 Free PMC article.
-
The presence of the casein kinase II phosphorylation sites of Vpu enhances the CD4(+) T cell loss caused by the simian-human immunodeficiency virus SHIV(KU-lbMC33) in pig-tailed macaques.Virology. 2003 Sep 1;313(2):435-51. doi: 10.1016/s0042-6822(03)00339-8. Virology. 2003. PMID: 12954211
-
A variant macaque-tropic human immunodeficiency virus type 1 is resistant to alpha interferon-induced restriction in pig-tailed macaque CD4+ T cells.J Virol. 2013 Jun;87(12):6678-92. doi: 10.1128/JVI.00338-13. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552412 Free PMC article.
-
Stepping toward a macaque model of HIV-1 induced AIDS.Viruses. 2014 Sep 25;6(9):3643-51. doi: 10.3390/v6093643. Viruses. 2014. PMID: 25256394 Free PMC article. Review.
Cited by
-
The HIV-1 pandemic: does the selective sweep in chimpanzees mirror humankind's future?Retrovirology. 2013 May 24;10:53. doi: 10.1186/1742-4690-10-53. Retrovirology. 2013. PMID: 23705941 Free PMC article. Review.
-
In vivo Serial Passaging of Human-Simian Immunodeficiency Virus Clones Identifies Characteristics for Persistent Viral Replication.Front Microbiol. 2021 Nov 18;12:779460. doi: 10.3389/fmicb.2021.779460. eCollection 2021. Front Microbiol. 2021. PMID: 34867922 Free PMC article.
-
Commentary: Derivation of Simian Tropic HIV-1 Infectious Clone Reveals Virus Adaptation to a New Host.Front Cell Infect Microbiol. 2020 May 15;10:235. doi: 10.3389/fcimb.2020.00235. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32500043 Free PMC article. No abstract available.
-
Complete Genome Sequences of Human Immunodeficiency Type 1 Viruses Genetically Engineered To Be Tropic for Rhesus Macaques.Genome Announc. 2017 Sep 28;5(39):e01063-17. doi: 10.1128/genomeA.01063-17. Genome Announc. 2017. PMID: 28963223 Free PMC article.
-
Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models.Viruses. 2024 Oct 16;16(10):1618. doi: 10.3390/v16101618. Viruses. 2024. PMID: 39459950 Free PMC article. Review.
References
-
- Agy M. B., et al. 1992. Infection of Macaca nemestrina by human immunodeficiency virus type 1. Science 257:103–106 - PubMed
-
- Agy M. B., et al. 1997. Serial in vivo passage of HIV-1 infection in Macaca nemestrina. Virology 238:336–343 - PubMed
-
- Ambrose Z., KewalRamani V. N., Bieniasz P. D., Hatziioannou T. 2007. HIV/AIDS: in search of an animal model. Trends Biotechnol. 25:333–337 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

