Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3beta
- PMID: 21289184
- PMCID: PMC3711464
- DOI: 10.1523/JNEUROSCI.5704-10.2011
Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3beta
Abstract
Although it is well established that pharmacological inhibitors of classical histone deacetylases (HDACs) are protective in various in vivo models of neurodegenerative disease, the identity of the neurotoxic HDAC(s) that these inhibitors target to exert their protective effects has not been resolved. We find that HDAC3 is a protein with strong neurotoxic activity. Forced expression of HDAC3 induces death of otherwise healthy rat cerebellar granule neurons, whereas shRNA-mediated suppression of its expression protects against low-potassium-induced neuronal death. Forced expression of HDAC3 also promotes the death of rat cortical neurons and hippocampally derived HT22 cells, but has no effect on the viability of primary kidney fibroblasts or the HEK293 and HeLa cell lines. This suggests that the toxic effect of HDAC3 is cell selective and that neurons are sensitive to it. Neurotoxicity by HDAC3 is inhibited by treatment with IGF-1 as well as by the expression of a constitutively active form of Akt, an essential mediator of IGF-1 signaling. Protection against HDAC3-induced neurotoxicity is also achieved by the inhibition of GSK3β, a kinase inhibited by Akt that is widely implicated in the promotion of neurodegeneration in experimental models and in human pathologies. HDAC3 is directly phosphorylated by GSK3β, suggesting that the neuronal death-promoting action of GSK3β could be mediated through HDAC3 phosphorylation. In addition to demonstrating that HDAC3 has neurotoxic effects, our study identifies it as a downstream target of GSK3β.
Figures
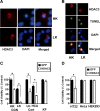
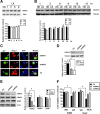
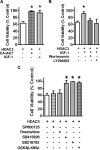
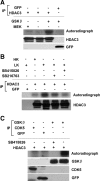
References
-
- Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. - PubMed
-
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. - PubMed
-
- Camins A, Verdaguer E, Folch J, Canudas AM, Pallàs M. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006;19:453–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
