Gateways of ventral and dorsal streams in mouse visual cortex
- PMID: 21289200
- PMCID: PMC3040111
- DOI: 10.1523/JNEUROSCI.3488-10.2011
Gateways of ventral and dorsal streams in mouse visual cortex
Abstract
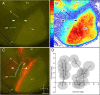
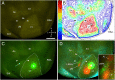
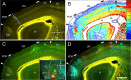
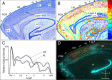
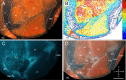
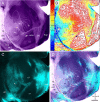
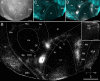
It is widely held that the spatial processing functions underlying rodent navigation are similar to those encoding human episodic memory (Doeller et al., 2010). Spatial and nonspatial information are provided by all senses including vision. It has been suggested that visual inputs are fed to the navigational network in cortex and hippocampus through dorsal and ventral intracortical streams (Whitlock et al., 2008), but this has not been shown directly in rodents. We have used cytoarchitectonic and chemoarchitectonic markers, topographic mapping of receptive fields, and pathway tracing to determine in mouse visual cortex whether the lateromedial field (LM) and the anterolateral field (AL), which are the principal targets of primary visual cortex (V1) (Wang and Burkhalter, 2007) specialized for processing nonspatial and spatial visual information (Gao et al., 2006), are distinct areas with diverse connections. We have found that the LM/AL border coincides with a change in type 2 muscarinic acetylcholine receptor expression in layer 4 and with the representation of the lower visual field periphery. Our quantitative analyses also show that LM strongly projects to temporal cortex as well as the lateral entorhinal cortex, which has weak spatial selectivity (Hargreaves et al., 2005). In contrast, AL has stronger connections with posterior parietal cortex, motor cortex, and the spatially selective medial entorhinal cortex (Haftig et al., 2005). These results support the notion that LM and AL are architecturally, topographically, and connectionally distinct areas of extrastriate visual cortex and that they are gateways for ventral and dorsal streams.
Figures



References
-
- Ahmed B, Anderson JC, Douglas RJ, Martin KAC, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. - PubMed
-
- Alloway KD. Information processing stream in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb Cortex. 2008;18:979–989. - PubMed
-
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 2010;31:2221–2233. - PubMed
-
- Beckstead RM. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol. 1979;184:43–61. - PubMed
-
- Benison AM, Rector DM, Barth DS. Hemispheric mapping of secondary somatosensory cortex in the rat. J Neurophysiol. 2007;97:200–2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
