Distinct macrophage phenotypes contribute to kidney injury and repair
- PMID: 21289217
- PMCID: PMC3029904
- DOI: 10.1681/ASN.2009060615
Distinct macrophage phenotypes contribute to kidney injury and repair
Abstract
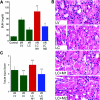
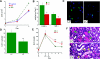
The ischemically injured kidney undergoes tubular cell necrosis and apoptosis, accompanied by an interstitial inflammatory cell infiltrate. In this study, we show that iNos-positive proinflammatory (M1) macrophages are recruited into the kidney in the first 48 hours after ischemia/reperfusion injury, whereas arginase 1- and mannose receptor-positive, noninflammatory (M2) macrophages predominate at later time points. Furthermore, depletion of macrophages before ischemia/reperfusion diminishes kidney injury, whereas depletion at 3 to 5 days after injury slows tubular cell proliferation and repair. Infusion of Ifnγ-stimulated, bone marrow-derived macrophages into macrophage-depleted mice at the time of kidney reperfusion restored injury to the level seen without macrophage depletion, suggesting that proinflammatory macrophages worsen kidney damage. In contrast, the appearance of macrophages with the M2 phenotype correlated with the proliferative phase of kidney repair. In vitro studies showed that IFNγ-stimulated, proinflammatory macrophages begin to express markers of M2 macrophages when cocultured with renal tubular cells. Moreover, IL-4-stimulated macrophages with an M2 phenotype, but not IFNγ-stimulated proinflammatory macrophages, promoted renal tubular cell proliferation. Finally, tracking fluorescently labeled, IFNγ-stimulated macrophages that were injected after injury showed that inflammatory macrophages can switch to an M2 phenotype in the kidney at the onset of kidney repair. Taken together, these studies show that macrophages undergo a switch from a proinflammatory to a trophic phenotype that supports the transition from tubule injury to tubule repair.
Figures

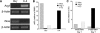


Comment in
-
Macrophages in kidney repair and regeneration.J Am Soc Nephrol. 2011 Feb;22(2):199-201. doi: 10.1681/ASN.2010121301. J Am Soc Nephrol. 2011. PMID: 21289208 No abstract available.
References
-
- Solez K, Morel-Maroger L, Sraer JD: The morphology of “acute tubular necrosis” in man: Analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 58: 362–376, 1979 - PubMed
-
- Bonventre JV: Pathophysiology of acute kidney injury: Roles of potential inhibitors of inflammation. Contrib Nephrol 156: 39–46, 2007 - PubMed
-
- Ibrahim S, Jacobs F, Zukin Y, Enriquez D, Holt D, Baldwin W, III, Sanfilippo F, Ratner LE: Immunohistochemical manifestations of unilateral kidney ischemia. Clin Transplant 10: 646–652, 1996 - PubMed
-
- Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, De Broe ME: T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 66: 491–496, 2004 - PubMed
-
- Beck-Schimmer B, Oertli B, Pasch T, Wuthrich RP: Hyaluronan induces monocyte chemoattractant protein-1 expression in renal tubular epithelial cells. J Am Soc Nephrol 9: 2283–2290, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

