BAX supports the mitochondrial network, promoting bioenergetics in nonapoptotic cells
- PMID: 21289292
- PMCID: PMC3118620
- DOI: 10.1152/ajpcell.00325.2010
BAX supports the mitochondrial network, promoting bioenergetics in nonapoptotic cells
Abstract
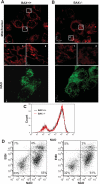
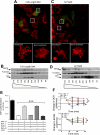
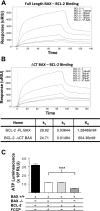
The dual functionality of the tumor suppressor BAX is implied by the nonapoptotic functions of other members of the BCL-2 family. To explore this, mitochondrial metabolism was examined in BAX-deficient HCT-116 cells as well as primary hepatocytes from BAX-deficient mice. Although mitochondrial density and mitochondrial DNA content were the same in BAX-containing and BAX-deficient cells, MitoTracker staining patterns differed, suggesting the existence of BAX-dependent functional differences in mitochondrial physiology. Oxygen consumption and cellular ATP levels were reduced in BAX-deficient cells, while glycolysis was increased. These results suggested that cells lacking BAX have a deficiency in the ability to generate ATP through cellular respiration. This conclusion was supported by detection of reduced citrate synthase activity in BAX-deficient cells. In nonapoptotic cells, a portion of BAX associated with mitochondria and a sequestered, protease-resistant form was detected. Inhibition of BAX with small interfering RNAs reduced intracellular ATP content in BAX-containing cells. Expression of either full-length or COOH-terminal-truncated BAX in BAX-deficient cells rescued ATP synthesis and oxygen consumption and reduced glycolytic activity, suggesting that this metabolic function of BAX was not dependent upon its COOH-terminal helix. Expression of BCL-2 in BAX-containing cells resulted in a subsequent loss of ATP measured, implying that, even under nonapoptotic conditions, an antagonistic interaction exists between the two proteins. These findings infer that a basal amount of BAX is necessary to maintain energy production via aerobic respiration.
Figures




References
-
- Cartron PF, Oliver L, Martin S, Moreau C, LeCabellec MT, Jezequel P, Meflah K, Vallette FM. The expression of a new variant of the pro-apoptotic molecule Bax, Baxpsi, is correlated with an increased survival of glioblastoma multiforme patients. Hum Mol Genet 11: 675–687, 2002 - PubMed
-
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol 16: 395–419, 1998 - PubMed
-
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424: 952–956, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

