Cladistic analysis of olfactory and vomeronasal systems
- PMID: 21290004
- PMCID: PMC3032080
- DOI: 10.3389/fnana.2011.00003
Cladistic analysis of olfactory and vomeronasal systems
Abstract
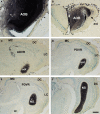
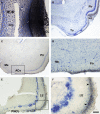
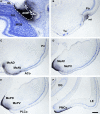
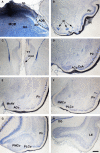
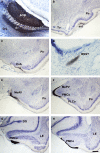
Most tetrapods possess two nasal organs for detecting chemicals in their environment, which are the sensory detectors of the olfactory and vomeronasal systems. The seventies' view that the olfactory system was only devoted to sense volatiles, whereas the vomeronasal system was exclusively specialized for pheromone detection was challenged by accumulating data showing deep anatomical and functional interrelationships between both systems. In addition, the assumption that the vomeronasal system appeared as an adaptation to terrestrial life is being questioned as well. The aim of the present work is to use a comparative strategy to gain insight in our understanding of the evolution of chemical "cortex." We have analyzed the organization of the olfactory and vomeronasal cortices of reptiles, marsupials, and placental mammals and we have compared our findings with data from other taxa in order to better understand the evolutionary history of the nasal sensory systems in vertebrates. The olfactory and vomeronsasal cortices have been re-investigated in garter snakes (Thamnophis sirtalis), short-tailed opossums (Monodelphis domestica), and rats (Rattus norvegicus) by tracing the efferents of the main and accessory olfactory bulbs using injections of neuroanatomical anterograde tracers (dextran-amines). In snakes, the medial olfactory tract is quite evident, whereas the main vomeronasal-recipient structure, the nucleus sphaericus is a folded cortical-like structure, located at the caudal edge of the amygdala. In marsupials, which are acallosal mammals, the rhinal fissure is relatively dorsal and the olfactory and vomeronasal cortices relatively expanded. Placental mammals, like marsupials, show partially overlapping olfactory and vomeronasal projections in the rostral basal telencephalon. These data raise the interesting question of how the telencephalon has been re-organized in different groups according to the biological relevance of chemical senses.
Keywords: amygdala; cortex; evolution; olfaction; olfactory bulb; vomeronasal.
Figures




References
-
- Abellan A., Legaz I., Vernier B., Retaux S., Medina L. (2009). Olfactory and amygdalar structures of the chicken ventral pallium based on the combinatorial expression patterns of LIM and other developmental regulatory genes. Comp. J. Neurol. 516, 166–186 - PubMed
-
- Alonso J. R., Covenas R., Lara J., Arevalo R., de Leon M., Aijon J. (1989). Tyrosine hydroxylase immunoreactivity in a subpopulation of granule cells in the olfactory bulb of teleost fish. Brain Behav. Evol. 34, 318–324 - PubMed
LinkOut - more resources
Full Text Sources

