Hyperthermia alters the interaction of proteins of the Mre11 complex in irradiated cells
- PMID: 21290468
- PMCID: PMC3075327
- DOI: 10.1002/cyto.a.20955
Hyperthermia alters the interaction of proteins of the Mre11 complex in irradiated cells
Abstract
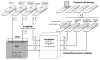
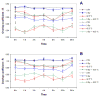
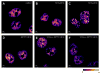
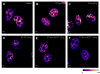
Radiosensitization of mammalian cells by heat is believed to involve the inhibition of repair of DNA double-strand breaks (DSBs). The Mre11 complex (composed of Mre11, Rad50, and Nbs1) is involved in DSB repair and forms foci at sites of radiation-induced DSBs. Heat induces the translocation of a significant amount of Mre11, Rad50, and Nbs1 from the nucleus to the cytoplasm, but little is known about how heat affects the integrity of the proteins still remaining in nuclei, or alters kinetics of formation/disappearance of DNA repair foci in heated, irradiated cells. Here, we show that hyperthermia alters the interaction between proteins of the Mre11 complex in irradiated human melanoma cells and inhibits the formation of repair foci. At various times after X-irradiation and/or heating (2 h at 41.5 or 42.5 °C), the cells were fixed and stained for Mre11, Rad50, and Nbs1. Colocalization of proteins and formation and disappearance of nuclear foci in heated and/or irradiated cells, determined using confocal microscopy, were compared. In heated, irradiated cells, focus formation was inhibited for 2-8 h, and colocalization of the proteins of the Mre11 complex was reduced for 12-24 h post-treatment. Colocalization was recovered in irradiated cells within 24 h after heating at 41.5 °C, but was inhibited longer after heating at 42.5 °C. The decreased colocalization in heated, irradiated cells suggests that there is a decrease in protein interaction, and Mre11 complexes in nuclei disassemble after heating. Such changes could be involved, at least in part, in heat radiosensitization and inhibition of DSB repair. Also, the kinetics of disassembly and reassembly of Mre11 complexes appears to be dependent upon treatment temperature.
Copyright © 2010 International Society for Advancement of Cytometry.
Figures




References
-
- Ben-Hur E, Elkind MM, Bronk BV. Thermally enhanced radioresponse of cultured Chinese hamster cells: inhibition of repair of sublethal damage and enhancement of lethal damage. Radiat Res. 1974;58:38–51. - PubMed
-
- Dewey WC, Hopwood LE, Sapareto LA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123:463–474. - PubMed
-
- Lepock JR, Cheng KH, Al-Qysi H, Kruuv J. Thermotropic lipid and protein transitions in Chinese hamster lung cell membranes: relationship to hyperthermic cell killing. Can J Biochem. 1983;61:421–427. - PubMed
-
- Lepock JR, Frey HE, Rodahl AM, Kruuv J. Thermal analysis of CHL V79 cells using differential scanning calorimetry: implications for hyperthermic cell killing and the heat shock response. J Cell Physiol. 1988;137:14–24. - PubMed
-
- Freeman ML, Borrelli MJ, Meredith MJ, Lepock JR. On the path to the heat shock response: destabilization and formation of partially folded protein intermediates, a consequence of protein thiol modification. Free Radic Biol Med. 1999;26:737–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

