Use of a centrifugal bioreactor for cartilaginous tissue formation from isolated chondrocytes
- PMID: 21290617
- PMCID: PMC3229169
- DOI: 10.1002/btpr.551
Use of a centrifugal bioreactor for cartilaginous tissue formation from isolated chondrocytes
Abstract
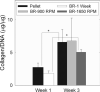
Although a centrifugal bioreactor (CCBR) supports high-density mammalian suspension cell cultures by balancing drag, buoyancy, and centrifugal forces, to date anchorage-dependent cultures have not been tried. Also, steady or intermittent hydrostatic pressures of 8 to 500 kPa, and shears of 0.02 to 1.4 N/m(2) can be simultaneously applied in the CCBR. This article demonstrates the use of a CCBR to stimulate chondrogenesis in a high-density culture. At 3 weeks, histological results show even distribution of glycosaminoglycan (GAG) and collagen, with 1,890 ± 270 cells/mm(2) cell densities that exceed those of 1,470 ± 270 in pellet cultures. Analysis of collagen content reveals similar levels for all treatment groups; 6.8 ± 3.5 and 5.0 ± 0.4 μg collagen/μg DNA for 0.07 and 0.26 MPa CCBR cultures, respectively, in contrast to 6.6 ± 1.9 values for control pellet cultures. GAG levels of 5.6 ± 1.5 and 4.1 ± 0.9 μg GAG /μg DNA are present for cultures stressed at 0.07 and 0.26 MPa, respectively, in comparison to control pellet cultures at the 8.4 ± 0.9 level. Although results to date have not revealed mechanical stress combinations that stimulate chondrogenesis over unstressed controls, system advantages include continuous culture at cell densities above those in the pellet, precise medium control, the ability to independently vary multiple mechanical stresses over a broad range, and the flexibility for integration of scaffold features for future chondrogenesis stimulation studies.
Copyright © 2011 American Institute of Chemical Engineers (AIChE).
Figures




References
-
- Dimicco MA, Kisiday JD, Gong H, Grodzinsky AJ. Structure of pericellular matrix around agarose-embedded chondrocytes. Osteoarthr Cartil. 2007;15:1207–1216. - PubMed
-
- Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. - PubMed
-
- Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, Schlegel J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthr Cartil. 2002;10:62–70. - PubMed
-
- Akmal M, Anand A, Anand B, Wiseman M, Goodship AE, Bentley G. The culture of articular chondrocytes in hydrogel constructs within a bioreactor enhances cell proliferation and matrix synthesis. J Bone Joint Surg Br. 2006;88:544–553. - PubMed
-
- Freyria AM, Yang Y, Chajra H, Rousseau CF, Ronziere MC, Herbage D, El Haj AJ. Optimization of dynamic culture conditions: effects on biosynthetic activities of chondrocytes grown in collagen sponges. Tissue Eng. 2005;11:674–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

