A conserved surface loop in type I dehydroquinate dehydratases positions an active site arginine and functions in substrate binding
- PMID: 21291284
- PMCID: PMC3062685
- DOI: 10.1021/bi102020s
A conserved surface loop in type I dehydroquinate dehydratases positions an active site arginine and functions in substrate binding
Abstract
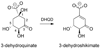
Dehydroquinate dehydratase (DHQD) catalyzes the third step in the biosynthetic shikimate pathway. We present three crystal structures of the Salmonella enterica type I DHQD that address the functionality of a surface loop that is observed to close over the active site following substrate binding. Two wild-type structures with differing loop conformations and kinetic and structural studies of a mutant provide evidence of both direct and indirect mechanisms of involvement of the loop in substrate binding. In addition to allowing amino acid side chains to establish a direct interaction with the substrate, closure of the loop necessitates a conformational change of a key active site arginine, which in turn positions the substrate productively. The absence of DHQD in humans and its essentiality in many pathogenic bacteria make the enzyme a target for the development of nontoxic antimicrobials. The structures and ligand binding insights presented here may inform the design of novel type I DHQD inhibiting molecules.
Figures

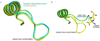
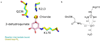
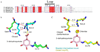
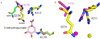
References
-
- Bentley R. The shikimate pathway--a metabolic tree with many branches. Crit Rev Biochem Mol Biol. 1990;25:307–384. - PubMed
-
- Benowitz AB, Hoover Jennifer L, Payne David J. Antibacterial Drug Discovery in the Age of Resistance. Microbe. 2010;5:390–396.
-
- Microbe GM, Shah DM. Amino-Acid Biosynthesis Inhibitors as Herbicides. Annu Rev Biochem. 1988;57:627–663. - PubMed
-
- Marques MR, Pereira JH, Oliveira JS, Basso LA, de Azevedo WF, Santos DS, Palma MS. The inhibition of 5-enolpyruvylshikimate-3-phosphate synthase as a model for development of novel antimicrobials. Curr Drug Targets. 2007;8:445–457. - PubMed
-
- Noble M, Sinha Y, Kolupaev A, Demin O, Earnshaw D, Tobin F, West J, Martin JD, Qiu CY, Liu WS, DeWolf WE, Tew D, Goryanin II. The kinetic model of the shikimate pathway as a tool to optimize enzyme assays for high-throughput screening. Biotechnol Bioeng. 2006;95:560–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

