Regulatory interactions of stress and reward on rat forebrain opioidergic and GABAergic circuitry
- PMID: 21291318
- PMCID: PMC3140340
- DOI: 10.3109/10253890.2010.531331
Regulatory interactions of stress and reward on rat forebrain opioidergic and GABAergic circuitry
Abstract
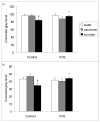
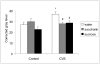
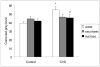
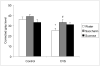
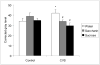
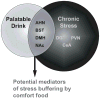
Palatable food intake reduces stress responses, suggesting that individuals may consume such ?comfort? food as self-medication for stress relief. The mechanism by which palatable foods provide stress relief is not known, but likely lies at the intersection of forebrain reward and stress regulatory circuits. Forebrain opioidergic and gamma-aminobutyric acid ergic signaling is critical for both reward and stress regulation, suggesting that these systems are prime candidates for mediating stress relief by palatable foods. Thus, the present study (1) determines how palatable ?comfort? food alters stress-induced changes in the mRNA expression of inhibitory neurotransmitters in reward and stress neurocircuitry and (2) identifies candidate brain regions that may underlie comfort food-mediated stress reduction. We used a model of palatable ?snacking? in combination with a model of chronic variable stress followed by in situ hybridization to determine forebrain levels of pro-opioid and glutamic acid decarboxylase (GAD) mRNA. The data identify regions within the extended amygdala, striatum, and hypothalamus as potential regions for mediating hypothalamic-pituitary-adrenal axis buffering following palatable snacking. Specifically, palatable snacking alone decreased pro-enkephalin-A (ENK) mRNA expression in the anterior bed nucleus of the stria terminalis (BST) and the nucleus accumbens, and decreased GAD65 mRNA in the posterior BST. Chronic stress alone increased ENK mRNA in the hypothalamus, nucleus accumbens, amygdala, and hippocampus; increased dynorphin mRNA in the nucleus accumbens; increased GAD65 mRNA in the anterior hypothalamus and BST; and decreased GAD65 mRNA in the dorsal hypothalamus. Importantly, palatable food intake prevented stress-induced gene expression changes in subregions of the hypothalamus, BST, and nucleus accumbens. Overall, these data suggest that complex interactions exist between brain reward and stress pathways and that palatable snacking can mitigate many of the neurochemical alterations induced by chronic stress.
Conflict of interest statement
Figures


References
-
- Ahima RS, Garcia MM, Harlan RE. Glucocorticoid regulation of preproenkephalin gene expression in the rat forebrain. Brain Res Mol Brain Res. 1992;16:119–127. - PubMed
-
- Brandao ML, Di Scala G, Bouchet MJ, Schmitt P. Escape behavior produced by the blockade of glutamic acid decarboxylase (GAD) in mesencephalic central gray or medial hypothalamus. Pharmacol Biochem Behav. 1986;24:497–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
