Performance of a new reusable insulin pen
- PMID: 21291331
- PMCID: PMC3103836
- DOI: 10.1089/dia.2010.0174
Performance of a new reusable insulin pen
Abstract
Background: The aim of this multinational (Canada, France, Germany, United Kingdom, and United States), task and interview-based study was to compare the ease of use and performance of the ClikSTAR® (sanofi-aventis, Paris, France) insulin pen with other commonly used reusable pens based on participant and interviewer assessments.
Methods: People with diabetes (n = 654) were asked to demonstrate four pens consecutively-ClikSTAR, Lilly Luxura ® (Eli Lilly, Indianapolis, IN), and NovoPen ® 3 and 4 (Novo Nordisk, Bagsvaerd, Denmark)-according to the respective instruction manuals. The endpoint was assessed by a rating from the participants and the interviewer. While the participants focused on the pen's ease of use, the interviewer considered the participants' difficulty in preparing and delivering a 40-unit dose and their requirement for help.
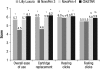
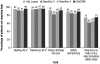
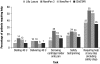
Results: Twenty percent of U.S. participants and 24% of participants from the other countries had type 1 diabetes. Approximately 50% of participants in each group had prior insulin pen experience. A higher proportion of participants, including those with dexterity or visual impairments, reported ClikSTAR as easier to use than other pens (P < 0.05). Participants using ClikSTAR did not experience any difficulty in completing the tasks. The proportion of participants not requiring help in completing the tasks with ClikSTAR was rated as numerically higher than, or similar to, that observed with Lilly Luxura or NovoPen 3 or 4 (75%, 74%, 62%, and 65%, respectively). According to participants, ClikSTAR and NovoPen 4 emerged as the most highly rated pens.
Conclusions: In comparison with other pens, ClikSTAR was significantly easier to use, which, when taken together with overall performance, meets the need of people with diabetes.
Figures

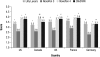
References
-
- Coscelli C. Lostia S. Lunetta M. Nosari I. Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28:173–177. - PubMed
-
- D'Eliseo P. Blaauw J. Milicevic Z. Wyatt J. Ignaut DA. Malone JK. Patient acceptability of a new 3.0 ml pre-filled insulin pen. Curr Med Res Opin. 2000;16:125–133. - PubMed
-
- Haak T. Edelman S. Walter C. Lecointre B. Spollett G. Comparison of usability and patient preference for the new disposable insulin device Solostar versus Flexpen, Lilly disposable pen, and a prototype pen: an open-label study. Clin Ther. 2007;29:650–660. - PubMed
-
- Korytkowski M. Bell D. Jacobsen C. Suwannasari R. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–2848. - PubMed
-
- Summers KH. Szeinbach SL. Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26:1498–1505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
