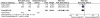The Early External Cephalic Version (ECV) 2 Trial: an international multicentre randomised controlled trial of timing of ECV for breech pregnancies
- PMID: 21291506
- PMCID: PMC3085121
- DOI: 10.1111/j.1471-0528.2010.02837.x
The Early External Cephalic Version (ECV) 2 Trial: an international multicentre randomised controlled trial of timing of ECV for breech pregnancies
Abstract
Objective: To investigate whether initiating external cephalic version (ECV) earlier in pregnancy might increase the rate of successful ECV procedures, and be more effective in decreasing the rate of non-cephalic presentation at birth and of caesarean section.
Design: An unblinded multicentred randomised controlled trial.
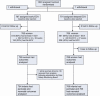
Setting: A total of 1543 women were randomised from 68 centres in 21 countries.
Population: Women with a singleton breech fetus at a gestational age of 33(0/7) weeks (231 days) to 35(6/7) weeks (251 days) of gestation were included.
Methods: Participants were randomly assigned to having a first ECV procedure between the gestational ages of 34(0/7) (238 days) and 35(6/7) weeks of gestation (early ECV group) or at or after 37(0/7) (259 days) weeks of gestation (delayed ECV group).
Main outcome measures: The primary outcome was the rate of caesarean section; the secondary outcome was the rate of preterm birth.
Results: Fewer fetuses were in a non-cephalic presentation at birth in the early ECV group (314/765 [41.1%] versus 377/768 [49.1%] in the delayed ECV group; relative risk [RR] 0.84, 95% CI 0.75, 0.94, P=0.002). There were no differences in rates of caesarean section (398/765 [52.0%] versus 430/768 [56.0%]; RR 0.93, 95% CI 0.85, 1.02, P=0.12) or in risk of preterm birth (50/765 [6.5%] versus 34/768 [4.4%]; RR 1.48, 95% CI 0.97, 2.26, P=0.07) between groups.
Conclusion: External cephalic version at 34-35 weeks versus 37 or more weeks of gestation increases the likelihood of cephalic presentation at birth but does not reduce the rate of caesarean section and may increase the rate of preterm birth.
© 2011 The Authors BJOG An International Journal of Obstetrics and Gynaecology © 2011 RCOG.
Figures
Comment in
-
Early versus late external cephalic version.BJOG. 2011 Sep;118(10):1272; author reply 1272-3. doi: 10.1111/j.1471-0528.2011.03058.x. BJOG. 2011. PMID: 21834883 No abstract available.
-
Early (at 34-35 weeks) external cephalic version reduced the risk of non-cephalic (breech) presentation at birth but has no effect on risk of caesarean section.Evid Based Med. 2012 Apr;17(2):45-6. doi: 10.1136/ebm.2011.100125. Epub 2011 Sep 26. Evid Based Med. 2012. PMID: 21949255 No abstract available.
References
-
- Hutton EK, Hannah ME, Barrett J. Use of external cephalic version for breech pregnancy and mode of delivery for breech and twin pregnancy: a survey of Canadian practitioners. J Obstet Gynaecol Can. 2002;24:804–10. - PubMed
-
- Goffinet F, Carayol M, Foidart JM, Alexander S, Uzan S, Subtil D, et al. for the PREMODA Study Group Is planned vaginal delivery for breech presentation at term still an option? Results of an observational prospective survey in France and Belgium. Am J Obstet Gynecol. 2006;194:1002–11. - PubMed
-
- Hofmeyr GJ, Kulier R. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2005 Issue 1. Art. No.: CD000083. DOI: 10.1002/14651858.CD000083. - DOI - PubMed
-
- Impey LWM, Hofmeyr GJ. External Cephalic Version and Reducing the Incidence of Breech Presentation. London: RCOG Press; 2006. Green Top Guidelines No.20a.
-
- American College of Obstetrics and Gynecology (ACOG) Clinical management guidelines for obstetrician–gynecologists: External Cephalic Version. ACOG Practice Bull. 2000;13:380–5. (reaffirmed 2009)
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources