Plasmodium vivax recurrence following falciparum and mixed species malaria: risk factors and effect of antimalarial kinetics
- PMID: 21292666
- PMCID: PMC3060895
- DOI: 10.1093/cid/ciq249
Plasmodium vivax recurrence following falciparum and mixed species malaria: risk factors and effect of antimalarial kinetics
Abstract
Background: Plasmodium vivax malaria commonly follows treatment of falciparum malaria in regions of co-endemicity. This is an important cause of preventable morbidity.
Methods: We examined the factors contributing to the risk of recurrence of P. vivax infection after treatment of acute falciparum malaria in a series of clinical trials conducted on the Thai-Myanmar border from 1991 through 2005.
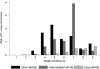
Results: Overall, 10,549 patients (4960 children aged <15 years and 5589 adults) were treated for falciparum malaria; of these patients, 9385 (89.0%) had Plasmodium falciparum monoinfection and 1164 (11.0%) had mixed P. falciparum/P. vivax infections according to microscopic examinations performed at screening. The cumulative proportion of patients with P. falciparum infection recurrence by day 63 was 21.5% (95% confidence interval [CI], 20.3%-22.8%), and the cumulative proportion with P. vivax infection recurrence was 31.5% (95% CI, 30.1%-33.0%). Significant risk factors for P. vivax infection recurrence were mixed infection at enrollment, male sex, younger age, lower hematocrit, higher asexual P. falciparum parasite density (P < .001 for all factors), and P. falciparum gametocytemia at enrollment (P = .001). By day 63, the cumulative risk of vivax malaria after P. falciparum monoinfection was 51.1% (95% CI, 46.1%-56.2%) after treatment with rapidly eliminated drugs (t(1/2) <1 day), 35.3% (95% CI, 31.8%-39.0%) after treatment with intermediate half-life drugs (t(1/2) 1-7 days), and 19.6% (95% CI, 18.1%-21.3%) after treatment with slowly eliminated drugs (t(1/2) > 7 days) (P < .001, by test for trend). Artemisinin-based combinations containing mefloquine or piperaquine, compared with the artemether-lumefantrine and artesunate-atovaquone-proguanil combinations, were associated with a 3.6-fold to 4.2-fold lower adjusted hazard ratio for P. vivax infection recurrence within 63 days after pure or mixed P. falciparum infections (P < .001, for comparisons with artesunate-mefloquine).
Conclusions: On the Thai-Myanmar border, P. vivax is the most common cause of parasitological failure after treatment for falciparum malaria. Slowly eliminated antimalarials reduce the risk of early P. vivax infection recurrence.
Figures
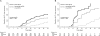

Comment in
-
Radical cure: the case for anti-relapse therapy against all malarias.Clin Infect Dis. 2011 Mar 1;52(5):621-3. doi: 10.1093/cid/ciq258. Clin Infect Dis. 2011. PMID: 21292667 Free PMC article. No abstract available.
References
-
- Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet. 1987;2:1052–5. - PubMed
-
- Ashley EA, Krudsood S, Phaiphun L, et al. Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J Infect Dis. 2004;190:1773–82. - PubMed
-
- Karunajeewa HA, Mueller I, Senn M, et al. A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med. 2008;359:2545–7. - PubMed
-
- Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–40. - PubMed

