Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009
- PMID: 21292718
- PMCID: PMC3033439
- DOI: 10.1136/bmj.c7297
Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009
Abstract
Objective: To assess the effectiveness of the pandemic influenza A/H1N1 vaccine used in Canada during autumn 2009.
Design: Test negative incident case-control study based on sentinel physician surveillance system.
Setting: Community based clinics contributing to sentinel networks in British Columbia, Alberta, Ontario, and Quebec, Canada.
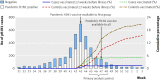
Participants: 552 patients who presented to a sentinel site within seven days of onset of influenza-like illness during the primary analysis period between 8 November and 5 December 2009; participants were mostly (>80%) children and adults under 50 years old.
Interventions: Monovalent AS03 adjuvanted pandemic influenza A/H1N1 vaccine as the predominant formulation (>95%) distributed in Canada.
Main outcome measures: Vaccine effectiveness calculated as 1-(odds ratio for influenza in vaccinated (received pandemic H1N1 vaccine at least two weeks before onset of influenza-like illness) versus unvaccinated participants), with adjustment for age, comorbidity, province, timeliness of specimen collection, and week of illness onset. Sensitivity analyses explored the influence of varying analysis periods between 1 November and 31 December, receipt of trivalent seasonal influenza vaccine, and restriction to participants without comorbidity.
Results: During the primary analysis period, pandemic H1N1 was detected by reverse transcription polymerase chain reaction in 209/552 (38%) participants; rates were highest in children and young adults (40%) and lowest in people aged 65 or over (9%). Among the 209 cases, 35 (17%) reported comorbidity compared with 80/343 (23%) controls. Two (1%) cases had received pandemic H1N1 vaccine at least two weeks before the onset of illness, compared with 58/343 (17%) controls, all single dose. Adjusted vaccine effectiveness overall was 93% (95% confidence interval 69% to 98%). High estimates of vaccine protection-generally at least 90%-were maintained across most sensitivity analyses.
Conclusions: Although limited by a small number of vaccine failures, this study suggests that the monovalent AS03 adjuvanted vaccine used in Canada during autumn 2009 was highly effective in preventing medically attended, laboratory confirmed pandemic H1N1 illness, with reference in particular to a single dose in children and young adults.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Pandemic influenza vaccines.BMJ. 2011 Feb 8;342:d545. doi: 10.1136/bmj.d545. BMJ. 2011. PMID: 21303882 No abstract available.
References
-
- GlaxoSmithKline Inc. Product information leaflet: Arepanrix™ H1N1. 2010. www.gsk.ca/english/docs-pdf/Arepanrix_2010.pdf.
-
- Committee for Proprietary Medicinal Products. Note for guidance on harmonization of requirements for influenza vaccines. CPMP/BWP/214/96 (circular no 96-0666):1-22. 1997. www.emea.europa.eu/pdfs/human/bwp/021496en.pdf.
-
- GlaxoSmithKline Inc. Results summaries: H1N1 Pandemic influenza vaccine. GSK study no 113482 (FLU Q-PAN H1N1-003 PRI). 2010. www.gsk-clinicalstudyregister.com/result_comp_list.jsp?compound=H1N1+Pan....
-
- GlaxoSmithKline Inc. Results summaries: H1N1 Pandemic influenza vaccine. GSK study no 113847 (FLU Q-PAN H1N1-0029). 2010. www.gsk-clinicalstudyregister.com/result_comp_list.jsp?compound=H1N1+Pan....
-
- Waddington CS, Walker WT, Oeser C, Reiner A, John T, Wilkins S, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ 2010;340:c2649. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
