Influence of oral and craniofacial dimensions on mandibular advancement splint treatment outcome in patients with obstructive sleep apnea
- PMID: 21292761
- PMCID: PMC5989786
- DOI: 10.1378/chest.10-2224
Influence of oral and craniofacial dimensions on mandibular advancement splint treatment outcome in patients with obstructive sleep apnea
Abstract
Background: Mandibular advancement splints (MASs) can effectively treat obstructive sleep apnea (OSA); however, no validated and reliable prediction method for treatment outcome currently exists. The efficacy of MAS may relate to anatomic factors, including craniofacial size and upper-airway soft-tissue volume and anatomic balance between them. We aimed to assess whether craniofacial and oral measurements are associated with MAS treatment outcome.
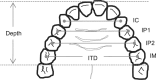
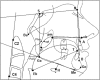
Methods: Dental impressions and lateral cephalometric radiographs were obtained from patients with OSA prior to commencing MAS treatment. Intertooth distances and palatal depths were measured on dental casts, and standard cephalometric analysis was performed with the addition of cross-sectional area (CSA) of the tongue and bony oral enclosure. Treatment outcome was determined by polysomnography.
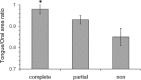
Results: Of 53 patients, 25 were complete responders (posttreatment apnea-hypopnea index [AHI] < 5/h), 17 were partial responders (≥ 50% AHI reduction), and 11 were nonresponders (< 50% AHI reduction). Cephalometric analyses did not reveal any significant differences between responders and nonresponders. Oral cavity measurements or CSA did not differ with treatment outcome; however, there was a trend toward a larger tongue CSA in complete vs partial and nonresponders (39.5 ± 1.3 cm(2) vs 35.5 ± 0.5 cm(2), P = .09). Tongue/oral enclosure CSA ratio, indicating a larger tongue for a given oral cavity size, was greater in complete responders (P = .012, n = 30).
Conclusions: Oral dimensions do not appear to differ between patients who respond and those who do not respond to MAS treatment. However, the larger tongue for a given oral cavity size in responders suggests that MAS may help to correct anatomic imbalance. Further research to assess whether the ratio between tongue and bony oral enclosure size may be useful in selecting patients for MAS treatment is warranted.
Figures

References
-
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. - PubMed
-
- McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 pt 1):1108–1114. - PubMed
-
- Weaver TE, Kribbs NB, Pack AI. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. - PubMed
-
- Kribbs NB, Pack AI, Kline LR. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. - PubMed
-
- Chan AS, Lee RW, Cistulli PA. Non-positive airway pressure modalities: mandibular advancement devices/positional therapy. Proc Am Thorac Soc. 2008;5(2):179–184. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

