P2X7 receptors trigger ATP exocytosis and modify secretory vesicle dynamics in neuroblastoma cells
- PMID: 21292765
- PMCID: PMC3064193
- DOI: 10.1074/jbc.M110.139410
P2X7 receptors trigger ATP exocytosis and modify secretory vesicle dynamics in neuroblastoma cells
Abstract
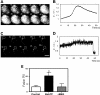
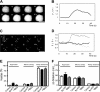
Previously, we reported that purinergic ionotropic P2X7 receptors negatively regulate neurite formation in Neuro-2a (N2a) mouse neuroblastoma cells through a Ca(2+)/calmodulin-dependent kinase II-related mechanism. In the present study we used this cell line to investigate a parallel though faster P2X7 receptor-mediated signaling pathway, namely Ca(2+)-regulated exocytosis. Selective activation of P2X7 receptors evoked exocytosis as assayed by high resolution membrane capacitance measurements. Using dual-wavelength total internal reflection microscopy, we have observed both the increase in near-membrane Ca(2+) concentration and the exocytosis of fluorescently labeled vesicles in response to P2X7 receptor stimulation. Moreover, activation of P2X7 receptors also affects vesicle motion in the vertical and horizontal directions, thus, involving this receptor type in the control of early steps (docking and priming) of the secretory pathway. Immunocytochemical and RT-PCR experiments evidenced that N2a cells express the three neuronal SNAREs as well as vesicular nucleotide and monoamine (VMAT-1 and VMAT-2) transporters. Biochemical measurements indicated that ionomycin induced a significant release of ATP from N2a cells. Finally, P2X7 receptor stimulation and ionomycin increased the incidence of small transient inward currents, reminiscent of postsynaptic quantal events observed at synapses. Small transient inward currents were dependent on extracellular Ca(2+) and were abolished by Brilliant Blue G, suggesting they were mediated by P2X7 receptors. Altogether, these results suggest the existence of a positive feedback mechanism mediated by P2X7 receptor-stimulated exocytotic release of ATP that would act on P2X7 receptors on the same or neighbor cells to further stimulate its own release and negatively control N2a cell differentiation.
Figures




References
-
- Burnstock G., Kennedy C. (1985) Gen. Pharmacol. 16, 433–440 - PubMed
-
- von Kügelgen I., Wetter A. (2000) Naunyn-Schmiedebergs Arch. Pharmacol. 362, 310–323 - PubMed
-
- Khakh B. S., Burnstock G., Kennedy C., King B. F., North R. A., Séguéla P., Voigt M., Humphrey P. P. (2001) Pharmacol. Rev. 53, 107–118 - PubMed
-
- North R. A. (2002) Physiol. Rev. 82, 1013–1067 - PubMed
-
- Jarvis M. F., Khakh B. S. (2009) Neuropharmacology 56, 208–215 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

