Ataxin-2 intermediate-length polyglutamine expansions in European ALS patients
- PMID: 21292779
- PMCID: PMC3071667
- DOI: 10.1093/hmg/ddr045
Ataxin-2 intermediate-length polyglutamine expansions in European ALS patients
Abstract
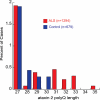
Amyotrophic lateral sclerosis (ALS) is a fatal adult-onset neurodegenerative disease primarily affecting motor neurons. We recently identified intermediate-length polyglutamine (polyQ) expansions (27-33 Qs) in ataxin 2 as a genetic risk factor for sporadic ALS in North American ALS patients. To extend these findings, we assessed the ataxin 2 polyQ repeat length in 1294 European ALS patients and 679 matched healthy controls. We observed a significant association between polyQ expansions and ALS (>30 Qs; P= 6.2 × 10(-3)). Thus, intermediate-length ataxin 2 polyQ repeat expansions are associated with increased risk for ALS also in the European cohort. The specific polyQ length cutoff, however, appears to vary between different populations, with longer repeat lengths showing a clear association. Our findings support the hypothesis that ataxin 2 plays an important role in predisposing to ALS and that polyQ expansions in ataxin 2 are a significant risk factor for the disease.
Figures
References
-
- Rosen D., Siddique T., Patterson D., Figlewicz D., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J., Deng H., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi:10.1038/362059a0. - DOI - PubMed
-
- Eisen A., Mezei M.M., Stewart H.G., Fabros M., Gibson G., Andersen P.M. SOD1 gene mutations in ALS patients from British Columbia, Canada: clinical features, neurophysiology and ethical issues in management. Amyotroph. Lateral. Scler. 2008;9:108–119. doi:10.1080/17482960801900073. - DOI - PubMed
-
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi:10.1126/science.1134108. - DOI - PubMed
-
- Pesiridis G.S., Lee V.M., Trojanowski J.Q. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Hum. Mol. Genet. 2009;18:R156–162. doi:10.1093/hmg/ddp303. - DOI - PMC - PubMed
-
- Kwiatkowski T.J., Jr, Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi:10.1126/science.1166066. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous