Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation
- PMID: 21292788
- PMCID: PMC3112017
- DOI: 10.1093/cvr/cvr042
Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation
Abstract
Aims: Vascular peroxidase 1 (VPO1) is a newly identified haem-containing peroxidase that catalyses the oxidation of a variety of substrates by hydrogen peroxide (H(2)O(2)). Considering the well-defined effects of H(2)O(2) on the vascular remodelling during hypertension, and that VPO1 can utilize H(2)O(2) generated from co-expressed NADPH oxidases to catalyse peroxidative reactions, the aims of this study were to determine the potential role of VPO1 in vascular remodelling during hypertension.
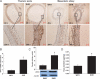
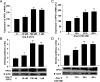
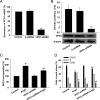
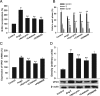
Methods and results: The vascular morphology and the expression of VPO1 in arterial tissues of spontaneously hypertensive rats and Wistar-Kyoto rats were assessed. The VPO1 expression was significantly increased concomitantly with definite vascular remodelling assessed by evaluating the media thickness, lumen diameter, media thickness-to-lumen diameter ratio and mean nuclear area in artery media in spontaneously hypertensive rats. In addition, in cultured rat aortic smooth muscle cells we found that the angiotensin II-mediated cell proliferation was inhibited by knockdown of VPO1 using small hairpin RNA. Moreover, the NADPH oxidase inhibitor, apocynin, and the hydrogen peroxide scavenger, catalase, but not the ERK1/2 inhibitor, PD98059, attenuated angiotensin II-mediated up-regulation of VPO1 and generation of hypochlorous acid.
Conclusion: VPO1 is a novel regulator of vascular smooth muscle cell proliferation via NADPH oxidase-H(2)O(2)-VPO1-hypochlorous acid-ERK1/2 pathways, which may contribute to vascular remodelling in hypertension.
Figures
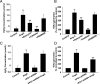
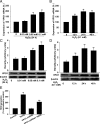
Comment in
-
Vascular peroxidase 1/peroxidasin: a complex protein with a simple function?Cardiovasc Res. 2011 Jul 1;91(1):1-2. doi: 10.1093/cvr/cvr120. Epub 2011 Apr 20. Cardiovasc Res. 2011. PMID: 21508041 No abstract available.
References
-
- Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi:10.1056/NEJM199405193302008. - DOI - PubMed
-
- Dzau VJ, Gibbons GH, Morishita R, Pratt RE. New perspectives in hypertention research: potentials of vascular biology. Hypertension. 1994;23:1132–1140. - PubMed
-
- Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. - PubMed
-
- Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, et al. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi:10.1161/CIRCRESAHA.107.163253. - DOI - PMC - PubMed
-
- Blanc A, Pandey NR, Srivastava AK. Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by H2O2 in vascular smooth muscle cells: potential involvement in vascular disease. Int J Mol Med. 2003;11:229–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

