Multi-scale lung modeling
- PMID: 21292842
- PMCID: PMC3098667
- DOI: 10.1152/japplphysiol.01289.2010
Multi-scale lung modeling
Abstract
Multi-scale modeling of biological systems has recently become fashionable due to the growing power of digital computers as well as to the growing realization that integrative systems behavior is as important to life as is the genome. While it is true that the behavior of a living organism must ultimately be traceable to all its components and their myriad interactions, attempting to codify this in its entirety in a model misses the insights gained from understanding how collections of system components at one level of scale conspire to produce qualitatively different behavior at higher levels. The essence of multi-scale modeling thus lies not in the inclusion of every conceivable biological detail, but rather in the judicious selection of emergent phenomena appropriate to the level of scale being modeled. These principles are exemplified in recent computational models of the lung. Airways responsiveness, for example, is an organ-level manifestation of events that begin at the molecular level within airway smooth muscle cells, yet it is not necessary to invoke all these molecular events to accurately describe the contraction dynamics of a cell, nor is it necessary to invoke all phenomena observable at the level of the cell to account for the changes in overall lung function that occur following methacholine challenge. Similarly, the regulation of pulmonary vascular tone has complex origins within the individual smooth muscle cells that line the blood vessels but, again, many of the fine details of cell behavior average out at the level of the organ to produce an effect on pulmonary vascular pressure that can be described in much simpler terms. The art of multi-scale lung modeling thus reduces not to being limitlessly inclusive, but rather to knowing what biological details to leave out.
Figures
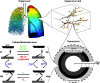
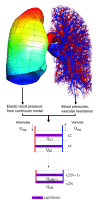
Comment in
-
Emergent behavior in lung structure and function.J Appl Physiol (1985). 2011 Apr;110(4):1109-10. doi: 10.1152/japplphysiol.00179.2011. Epub 2011 Feb 10. J Appl Physiol (1985). 2011. PMID: 21310887 No abstract available.
References
-
- An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 29: 834–860, 2007. - PMC - PubMed
-
- Bates JH. A micromechanical model of lung tissue rheology. Ann Biomed Eng 26: 679–687, 1998. - PubMed
-
- Bates JH. A recruitment model of quasi-linear power-law stress adaptation in lung tissue. Ann Biomed Eng 35: 1165–1174, 2007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

