A sorting platform determines the order of protein secretion in bacterial type III systems
- PMID: 21292939
- PMCID: PMC3859126
- DOI: 10.1126/science.1201476
A sorting platform determines the order of protein secretion in bacterial type III systems
Abstract
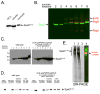
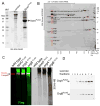
Bacterial type III protein secretion systems deliver effector proteins into eukaryotic cells in order to modulate cellular processes. Central to the function of these protein-delivery machines is their ability to recognize and secrete substrates in a defined order. Here, we describe a mechanism by which a type III secretion system from the bacterial enteropathogen Salmonella enterica serovar Typhimurium can sort its substrates before secretion. This mechanism involves a cytoplasmic sorting platform that is sequentially loaded with the appropriate secreted proteins. The sequential loading of this platform, facilitated by customized chaperones, ensures the hierarchy in type III protein secretion. Given the presence of these machines in many important pathogens, these findings can serve as the bases for the development of novel antimicrobial strategies.
Figures


Comment in
-
Microbiology. Establishing the secretion hierarchy.Science. 2011 Mar 4;331(6021):1147-8. doi: 10.1126/science.1203195. Science. 2011. PMID: 21385706 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

