Drosophila sperm motility in the reproductive tract
- PMID: 21293028
- PMCID: PMC3080424
- DOI: 10.1095/biolreprod.110.088773
Drosophila sperm motility in the reproductive tract
Abstract
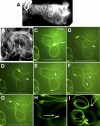
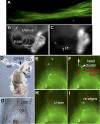
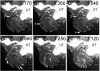
Motile cilia and flagella exhibit many waveforms as outputs of dynein activation sequences on the highly conserved axoneme. Motility change of sperm in the reproductive tract is difficult to study and remains an important area of investigation. Sperm typically execute a sinusoidal waveform. Increased viscosity in the medium induces somewhat unusual arc-line and helical waveforms in some sperm. However, whether the latter two waveforms occur in vivo is not known. Using green fluorescence protein imaging, we show that Drosophila sperm in the uterus move in circular foci via arc-line waves, predominantly in a tail-leading orientation. From the uterus, a small fraction of the sperm enters the seminal receptacle (SR) in parallel formations. After sperm storage and coincident with fertilization of the egg, the sperm exit the SR via head-leading helical waves. Consistent with the observed bidirectional movements, the sperm show the ability to propagate both base-to-tip and tip-to-base flagellar waves. Numerous studies have shown that sperm motility is regulated by intraflagellar calcium concentrations; in particular, the Pkd2 calcium channel has been shown to affect sperm storage. Our analyses here suggest that Pkd2 is required for the sperm to adopt the correct waveform and movement orientation during SR entry. A working model for the sperm's SR entry movement is proposed.
Figures





Similar articles
-
Fluorescent imaging of Drosophila melanogaster sperm in the reproductive tract: a new model of flagellar motility.Methods Enzymol. 2013;525:131-48. doi: 10.1016/B978-0-12-397944-5.00007-9. Methods Enzymol. 2013. PMID: 23522468
-
Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels.PLoS One. 2011;6(5):e20031. doi: 10.1371/journal.pone.0020031. Epub 2011 May 20. PLoS One. 2011. PMID: 21625494 Free PMC article.
-
Microscopic analysis of sperm movement: links to mechanisms and protein components.Microscopy (Oxf). 2018 Jun 1;67(3):144-155. doi: 10.1093/jmicro/dfy021. Microscopy (Oxf). 2018. PMID: 29741637 Review.
-
Resolving mechanisms of competitive fertilization success in Drosophila melanogaster.Science. 2010 Apr 16;328(5976):354-7. doi: 10.1126/science.1187096. Epub 2010 Mar 18. Science. 2010. PMID: 20299550
-
Sperm flagella: comparative and phylogenetic perspectives of protein components.Mol Hum Reprod. 2011 Aug;17(8):524-38. doi: 10.1093/molehr/gar034. Epub 2011 May 17. Mol Hum Reprod. 2011. PMID: 21586547 Review.
Cited by
-
A mosquito sperm's journey from male ejaculate to egg: Mechanisms, molecules, and methods for exploration.Mol Reprod Dev. 2016 Oct;83(10):897-911. doi: 10.1002/mrd.22653. Mol Reprod Dev. 2016. PMID: 27147424 Free PMC article. Review.
-
Metabolic Dysregulation and Sperm Motility in Male Infertility.Adv Exp Med Biol. 2022;1358:257-273. doi: 10.1007/978-3-030-89340-8_12. Adv Exp Med Biol. 2022. PMID: 35641874 Review.
-
The unity and diversity of the ciliary central apparatus.Philos Trans R Soc Lond B Biol Sci. 2020 Feb 17;375(1792):20190164. doi: 10.1098/rstb.2019.0164. Epub 2019 Dec 30. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31884923 Free PMC article.
-
Dissecting Fertility Functions of Drosophila Y Chromosome Genes with CRISPR.Genetics. 2020 Apr;214(4):977-990. doi: 10.1534/genetics.120.302672. Epub 2020 Feb 25. Genetics. 2020. PMID: 32098759 Free PMC article.
-
Bundle formation of sperm: Influence of environmental factors.Front Endocrinol (Lausanne). 2022 Oct 10;13:957684. doi: 10.3389/fendo.2022.957684. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36299459 Free PMC article.
References
-
- Eisenbach M, Giojalas LC. Sperm guidance in mammals—an unpaved road to the egg. Nat Rev Mol Cell Biol 2006; 7: 276 285 - PubMed
-
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 2008; 52: 455 462 - PubMed
-
- Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Fertilization defects in sperm from mice lacking fertilin beta. Science 1998; 281: 1857 1859 - PubMed
-
- Ikawa M, Nakanishi T, Yamada S, Wada I, Kominami K, Tanaka H, Nozaki M, Nishimune Y, Okabe M. Calmegin is required for fertilin alpha/beta heterodimerization and sperm fertility. Dev Biol 2001; 240: 254 261 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

