Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors
- PMID: 21293057
- PMCID: PMC3026735
- DOI: 10.1172/JCI44740
Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors
Abstract
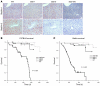
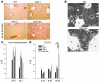
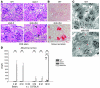
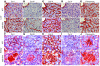
Tissue homeostasis and remodeling are processes that involve high turnover of biological macromolecules. Many of the waste molecules that are by-products or degradation intermediates of biological macromolecule turnover enter the circulation and are subsequently cleared by liver sinusoidal endothelial cells (LSEC). Besides the mannose receptor, stabilin-1 and stabilin-2 are the major scavenger receptors expressed by LSEC. To more clearly elucidate the functions of stabilin-1 and -2, we have generated mice lacking stabilin-1, stabilin-2, or both stabilin-1 and -2 (Stab1–/– Stab2–/– mice). Mice lacking either stabilin-1 or stabilin-2 were phenotypically normal; however, Stab1–/– Stab2–/– mice exhibited premature mortality and developed severe glomerular fibrosis, while the liver showed only mild perisinusoidal fibrosis without dysfunction. Upon kidney transplantation into WT mice, progression of glomerular fibrosis was halted, indicating the presence of profibrotic factors in the circulation of Stab1–/– Stab2–/– mice. While plasma levels of known profibrotic cytokines were unaltered, clearance of the TGF-β family member growth differentiation factor 15 (GDF-15) was markedly impaired in Stab1–/– Stab2–/– mice but not in either Stab1–/– or Stab2–/– mice, indicating that it is a common ligand of both stabilin-1 and stabilin-2. These data lead us to conclude that stabilin-1 and -2 together guarantee proper hepatic clearance of potentially noxious agents in the blood and maintain tissue homeostasis not only in the liver but also distant organs.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

