α3(V) collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues
- PMID: 21293061
- PMCID: PMC3026738
- DOI: 10.1172/JCI45096
α3(V) collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues
Abstract
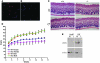
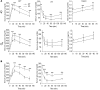
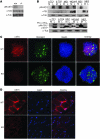
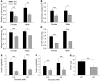
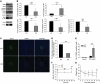
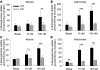
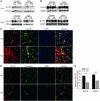
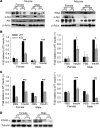
Collagen V, broadly expressed as α1(V)2 α2(V) heterotrimers that regulate collagen fibril geometry and strength, also occurs in some tissues, such as white adipose tissue (WAT), pancreatic islets, and skeletal muscle, as the poorly characterized α1(V) α2(V) α3(V) heterotrimer. Here, we investigate the role of α3(V) collagen chains by generating mice with a null allele of the α3(V) gene Col5a3 (Col5a3–/– mice). Female Col5a3–/– mice had reduced dermal fat and were resistant to high-fat diet–induced weight gain. Male and female mutant mice were glucose intolerant, insulin-resistant, and hyperglycemic, and these metabolic defects worsened with age. Col5a3–/– mice demonstrated decreased numbers of pancreatic islets, which were more susceptible to streptozotocin-induced apoptosis, and islets isolated from mutant mice displayed blunted glucose-stimulated insulin secretion. Moreover, Col5a3–/– WAT and skeletal muscle were defective in glucose uptake and mobilization of intracellular GLUT4 glucose transporter to the plasma membrane in response to insulin. Our results underscore the emerging view of the importance of ECM to the microenvironments that inform proper development/functioning of specialized cells, such as adipocytes, β cells, and skeletal muscle.
Figures

References
-
- Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95(pt 4):649–657. - PubMed
-
- Toriello HV, et al. A translocation interrupts the COL5A1 gene in a patient with Ehlers-Danlos syndrome and hypomelanosis of Ito. Nat Genet. 1996;13(3):361–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

