High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways
- PMID: 21293471
- PMCID: PMC4009937
- DOI: 10.1038/aps.2010.174
High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways
Abstract
Aim: To investigate whether high glucose stimulates the expression of inflammatory cytokines and the possible mechanisms involved.
Methods: ELISA and real-time PCR were used to determine the expression of the inflammatory factors, and a chemiluminescence assay was used to measure the production of reactive oxygen species (ROS).
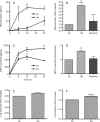
Results: Compared to low glucose (10 mmol/L), treatment with high glucose (35 mmol/L) increased the secretion of tumor necrosis factor (TNF)α and monocyte chemotactic protein-1 (MCP-1), but not interleukin (IL)-1β and IL-6, in a time-dependent manner in primary cultured rat microglia. The mRNA expression of TNFα and MCP-1 also increased in response to high glucose. This upregulation was specific to high glucose because it was not observed in the osmotic control. High-glucose treatment stimulated the formation of ROS. Furthermore, treatment with the ROS scavenger NAC significantly reduced the high glucose-induced TNFα and MCP-1 secretion. In addition, the nuclear factor kappa B (NF-κB) inhibitors MG132 and PDTC completely blocked the high glucose-induced TNFα and MCP-1 secretion.
Conclusion: We found that high glucose induces TNFα and MCP-1 secretion as well as mRNA expression in rat microglia in vitro, and this effect is mediated by the ROS and NF-κB pathways.
Figures



Similar articles
-
High glucose stimulates GRO secretion from rat microglia via ROS, PKC, and NF-kappaB pathways.J Neurosci Res. 2007 Nov 1;85(14):3150-9. doi: 10.1002/jnr.21421. J Neurosci Res. 2007. PMID: 17639599
-
Retinal neuronal MCP-1 induced by AGEs stimulates TNF-α expression in rat microglia via p38, ERK, and NF-κB pathways.Mol Vis. 2014 May 2;20:616-28. eCollection 2014. Mol Vis. 2014. PMID: 24826069 Free PMC article.
-
Hypoxia reduces the response of human adipocytes towards TNFα resulting in reduced NF-κB signaling and MCP-1 secretion.Int J Obes (Lond). 2012 Jul;36(7):986-92. doi: 10.1038/ijo.2011.200. Epub 2011 Oct 18. Int J Obes (Lond). 2012. PMID: 22005720
-
Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells.J Am Soc Nephrol. 2002 Apr;13(4):894-902. doi: 10.1681/ASN.V134894. J Am Soc Nephrol. 2002. PMID: 11912248
-
Angiotensin II and tumor necrosis factor-alpha synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-kappaB, p38, and reactive oxygen species.Am J Physiol Heart Circ Physiol. 2008 Jun;294(6):H2879-88. doi: 10.1152/ajpheart.91406.2007. Epub 2008 Apr 25. Am J Physiol Heart Circ Physiol. 2008. PMID: 18441197
Cited by
-
Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells.Int J Mol Sci. 2021 Nov 23;22(23):12668. doi: 10.3390/ijms222312668. Int J Mol Sci. 2021. PMID: 34884473 Free PMC article.
-
Niacin Suppresses Progression of Atherosclerosis by Inhibiting Vascular Inflammation and Apoptosis of Vascular Smooth Muscle Cells.Med Sci Monit. 2015 Dec 29;21:4081-9. doi: 10.12659/msm.895547. Med Sci Monit. 2015. PMID: 26712802 Free PMC article.
-
High glucose enhances HIV entry into T cells through upregulation of CXCR4.J Leukoc Biol. 2013 Oct;94(4):769-77. doi: 10.1189/jlb.0313142. Epub 2013 Aug 2. J Leukoc Biol. 2013. PMID: 23911867 Free PMC article.
-
A Comprehensive Profiling of Cellular Sphingolipids in Mammalian Endothelial and Microglial Cells Cultured in Normal and High-Glucose Conditions.Cells. 2022 Sep 30;11(19):3082. doi: 10.3390/cells11193082. Cells. 2022. PMID: 36231042 Free PMC article.
-
The Role of Osteopontin in Microglia Biology: Current Concepts and Future Perspectives.Biomedicines. 2022 Apr 3;10(4):840. doi: 10.3390/biomedicines10040840. Biomedicines. 2022. PMID: 35453590 Free PMC article. Review.
References
-
- Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. - PubMed
-
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. Faseb J. 2005;19:533–42. - PubMed
-
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

