Comparison of camel tear proteins between summer and winter
- PMID: 21293736
- PMCID: PMC3032277
Comparison of camel tear proteins between summer and winter
Abstract
Purpose: Proteins in the tear fluid have positive effects on maintaining the integrity and stabilization of the tear film, which is affected by several environmental factors. The aim of this study is to investigate seasonal variation of protein patterns in camel tears collected during the summer and winter season.
Methods: Tears from both eyes of 50 clinically normal camels (Camelus dromedarius) were collected in the summer (June-July) and in the winter (December-January) respectively. Pooled tear protein samples from two seasons were separated by SDS-PAGE and two-dimensional electrophoresis (2-DE). Protein spots of differential expression in two season gels were excised and subjected to in-gel digestion and identification by matrix assisted laser desorption/ionization-time of flight/time of flight-mass spectrum (MALDI-TOF/TOF-MS) analysis. Two differentially expressed proteins, lactoferrin (LF) and vitelline membrane outer layer protein 1 homolog (VMO1 homolog), were validated by western blotting.
Results: Thirteen well resolved bands were detected in SDS-PAGE gels of both summer and winter camel tears. By band densitometry, significantly higher intensities of band 6, 7, 11, and lower intensity of band 13 were observed in the summer group compared to the winter group. In 2-DE profiles of camel tears, four protein spots were found expressed differentially in two seasons. Further protein identification by MALDI-TOF/TOF-MS and confirmation by western blotting indicated that there was a significant decrease in LF (p=0.002) and an increase in VMO1 homolog (p=0.042) in tears in the summer compared to the winter.
Conclusions: The seasonal variation of camel tear fluids has been found in the composition of proteins, including LF and VMO1 homolog. This result will expand our knowledge of physiologic characteristics of tear fluids and establish a foundation for the mechanistic studies and clinical practices on ocular surface disorders.
Figures


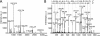

References
-
- Tiffany JM. The normal tear film. Dev Ophthalmol. 2008;41:1–20. - PubMed
-
- Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin Chim Acta. 2006;369:17–28. - PubMed
-
- Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. - PubMed
-
- Zhou L, Beuerman RW, Huang L, Barathi A, Foo YH, Li SFY, Chew FT, Tan D. Proteomic analysis of rabbit tear fluid: Defensin levels after an experimental corneal wound are correlated to wound closure. Proteomics. 2007;7:3194–206. - PubMed
-
- Wu KL, Zhang YL. Clinical application of tear proteomics: Present and future prospects. Proteomics Clin Appl. 2007;1:972–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
