A microfabricated deformability-based flow cytometer with application to malaria
- PMID: 21293801
- PMCID: PMC3366288
- DOI: 10.1039/c0lc00472c
A microfabricated deformability-based flow cytometer with application to malaria
Abstract
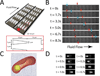
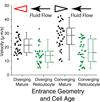
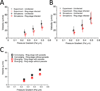
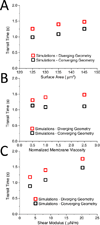
Malaria resulting from Plasmodium falciparum infection is a major cause of human suffering and mortality. Red blood cell (RBC) deformability plays a major role in the pathogenesis of malaria. Here we introduce an automated microfabricated "deformability cytometer" that measures dynamic mechanical responses of 10(3) to 10(4) individual RBCs in a cell population. Fluorescence measurements of each RBC are simultaneously acquired, resulting in a population-based correlation between biochemical properties, such as cell surface markers, and dynamic mechanical deformability. This device is especially applicable to heterogeneous cell populations. We demonstrate its ability to mechanically characterize a small number of P. falciparum-infected (ring stage) RBCs in a large population of uninfected RBCs. Furthermore, we are able to infer quantitative mechanical properties of individual RBCs from the observed dynamic behavior through a dissipative particle dynamics (DPD) model. These methods collectively provide a systematic approach to characterize the biomechanical properties of cells in a high-throughput manner.
This journal is © The Royal Society of Chemistry 2011
Figures
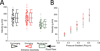


References
-
- McMillan D, Utterback N, La Puma J. Diabetes. 1978;27:895–901. - PubMed
-
- Cranston H, Boylan C, Carroll G, Sutera S, Williamson J, Gluzman I, Krogstad D. Science. 1983;233:400–403. - PubMed
-
- Mokken FC, Kedaria M, Henny CP, Hardeman M, Gleb A. Ann. Hematol. 1992;64:113–122. - PubMed
-
- WHO. World Malaria Report 2008. 2008
-
- Maier A, Cooke B, Cowman A, Tilley L. Nature Reviews Microbiology. 2009;7:341–354. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

